CRISPR Applications in Cancer Research Diagnosis and Treatment
- Aspirin: Study Finds Greater Benefits for These Colorectal Cancer Patients
- Cancer Can Occur Without Genetic Mutations?
- Statins Lower Blood Lipids: How Long is a Course?
- Warning: Smartwatch Blood Sugar Measurement Deemed Dangerous
- Mifepristone: A Safe and Effective Abortion Option Amidst Controversy
- Asbestos Detected in Buildings Damaged in Ukraine: Analyzed by Japanese Company
CRISPR Applications in Cancer Research Diagnosis and Treatment
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Nature Series Reviews: CRISPR Applications in Cancer Research Diagnosis and Treatment.
Since its inception, the CRISPR gene editing system has become a powerful tool for studying cellular function. It has a major impact on our understanding of cancer biology and continues to drive new discoveries that accelerate the development of cancer diagnostics and treatments.
Recently, a review article titled: CRISPR in cancer biology and therapy was published in the journal Nature Reviews Cancer , which systematically reviewed the latest progress of CRISPR system in cancer research, diagnosis and treatment.

The impact of CRISPR on cancer biology research
Precision medicine strategies for cancer rely on the discovery of genetic mutations that promote cancer growth, and CRISPR gene editing technology can rapidly and efficiently generate gene knockouts that modulate endogenous gene expression and replicate cancer-related genomic changes.
Due to the simplicity and efficiency of CRISPR technology, it has become routine to generate gene knockout mouse models . Moreover, by selectively introducing all components of the CRISPR gene editing system into certain somatic cells, tissue-specific cancer models can be generated .
For example, editing Tet2 , Runx1 , Dnmt3a , Nf1 and Smc3 genes in hematopoietic stem cells using CRISPR can stimulate acute myeloid leukemia.
Delivery of CRISPR to the liver, pancreas or lung enables rapid generation of cancer models with complex phenotypes.
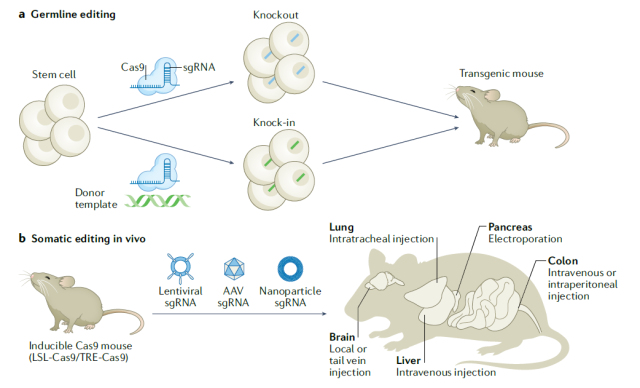
Generating Different Types of Cancer Models Using CRISPR Technology
The review authors note that perhaps the greatest impact CRISPR technology has had on cancer research is CRISPR screening.
Using a library of guide RNAs (gRNAs) targeting different genes in the genome, this screen can systematically knock out any gene in a cell line or organoid, and then observe the effect of the knockout on cancer cell growth or drug response.
Another important application of CRISPR in cancer research is tracking lineage changes in cancer cells .
A hallmark of cancer is its heterogeneity, with cancer cells accumulating genetic variations that lead to the creation of cell clones with distinct characteristics.
Understanding heterogeneity within tumors and tracking the generation and evolution of new clones has given scientists a more complete understanding of tumorigenesis.
At present, researchers have developed a variety of CRISPR-based tracking strategies that can find different cell clones in mixed cell populations containing multiple cell clones and track their dynamic changes over time.
CRISPR technology can introduce unique barcodes into cells that can be used to mark cancer cell lineages.
The latest CRISPR recording systems are able to introduce markers in the genome at specific times, and by analyzing these different markers, lineage relationships between different cancer cell clones can be constructed.


Different CRISPR strategies for tracing the clonal lineage of cancer cells
CRISPR Applications in Cancer Diagnostic Development
Early detection of cancer provides the best chance of curing cancer, and CRISPR technology can help develop more sensitive molecular diagnostic tools to aid in the early detection of cancer. CRISPR molecular diagnostic systems based on Cas12 and Cas13 have been used to identify cancer-related genetic mutations from patient tumor tissue biopsies.
They can emit a fluorescent signal by cleaving the RNA sequence carrying the fluorescent reporter protein after finding a specific oncogene mutation sequence.
This technology was used to produce rapid and sensitive tests for the new coronavirus during the COVID-19 virus pandemic.
The same platform can be used to generate highly sensitive personalized cancer discovery and monitoring systems.
In addition, the CRISPR system can be used to precisely cut DNA fragments in specific regions of the genome .
Compared with traditional random genome fragmentation, this method can enrich the DNA fragments of interest, which can be combined with next-generation gene sequencing. , gene mutations on specific genes can be found in very small sample sizes. This technique is currently being evaluated in clinical trials for the detection of p53 mutations in ovarian cancer.
CRISPR applications in cancer therapy
In cancer treatment, one of the main applications of CRISPR technology is to engineer immune cells to generate anti-cancer immunotherapies.
Multiple research teams have shown that targeting PD-1 expression in T cells using CRISPR gene editing can increase the anticancer activity of T cells. Such candidate therapies are already in clinical trials.
In addition, CRISPR gene editing can be used to knock out human leukocyte antigen (HLA) on the surface of endogenous T cell receptors and T cells from healthy donors when producing allogeneic “universal” CAR-T cell therapies , thereby reducing the risk of immune rejection and graft-versus-host disease caused by allogeneic cell infusion.
Moreover, using CRISPR/Cas9 technology, the CAR-expressing sequence can also be specifically inserted into the gene locus of the T cell receptor alpha constant region (TRAC) of the cell, resulting in the consistent expression of the CAR.
In vitro and in mouse models, CAR-T cells generated by this approach exhibited enhanced anticancer activity compared to conventional CAR-T cells.
 The many ways CRISPR can engineer T cells
The many ways CRISPR can engineer T cells
Using CRISPR gene editing to directly target the genetic variants that drive cancer , beyond in vitro engineered T-cell therapy, is an attractive, but also very difficult, challenge.
In theory, CRISPR gene editing can directly correct the genetic mutation that drives the cancer, or kill cancer cells that produce a specific genetic mutation, which can inhibit tumor growth.
However, this strategy needs to overcome multiple hurdles, including the delivery of tumor-specific therapies, and the need for high-efficiency gene editing.
Current preclinical studies have identified several strategies for tumor-specific gene editing. For example, CRISPR gene-editing systems that target oncogene fusions can selectively target tumor cells while disrupting the genetic mutations that promote tumor growth.
Another preclinical study put the transcription of the CRISPR-Cas13a system under the control of the NF-κB transcription factor.
Since NF-κB is overactivated in a variety of cancers, this strategy can specifically express the CRISPR-Cas13a system in cancer cells , Knock down the expression of oncogenes to achieve the effect of inhibiting the growth of cancer cells.
 Using NF-κB to control the expression of the CRISPR-Cas13a system, tumor-specific degradation of oncogenes
Using NF-κB to control the expression of the CRISPR-Cas13a system, tumor-specific degradation of oncogenes
In terms of delivery technology, lipid nanoparticles (LNP) have achieved great success in delivering 2019-nCoV mRNA.
The use of LNP to deliver Cas9-encoding mRNA and gRNA has effectively targeted PLK1 , a gene essential for mitosis , in proof-of-concept studies, reaching 70% in vivo gene editing efficiency in a mouse model of glioblastoma, causing apoptosis and inhibiting Tumor growth by 50% and improved survival by 30%.
Antibodies targeting tumor-specific antigens conjugated on the surface of LNPs also successfully drove dispersed tumors to selectively absorb LNPs, improving tumor-specific editing efficiency.
Taken as a whole, these preclinical studies show the potential of in vivo CRISPR gene editing for the treatment of cancer, although much effort is still required to translate CRISPR in vivo gene editing into viable clinical-stage anticancer therapies.
Limitations and Prospects
Although CRISPR has broad uses in cancer biology, the review authors note that further development of this technology, especially in clinical treatment, still needs to overcome several limitations. DNA double-strand breaks caused by Cas enzymes can trigger unintentional loss of DNA fragments and, in some cases, drive chromosome fragmentation (chromothripsis) , thereby affecting normal cell function.
DNA double-strand break damage caused by CRISPR technology may stimulate the p53 signaling pathway, resulting in cell death or selection of cells with reduced p53 function. In addition, the off-target editing potential of CRISPR systems has been a concern for researchers.
However, while these potential limitations are important limitations driving the further development of CRISPR technology, scientists have now developed tools to detect and minimize the occurrence of these events. The authors say they may not significantly hinder the clinical application of CRISPR technology.
In the next 5-10 years, CRISPR technology will truly enter the stage of clinical-stage therapy, and work in CAR-T therapy and other immune cell engineering heralds their role in the treatment of cancer.
In scientific research, CRISPR technology has begun to address many of the fundamental questions of cancer. By characterizing the role of individual genes in cancer cell behavior, it will provide the foundation for building the next generation of immunotherapies, uncovering the role of noncoding regions and regulatory elements in tumorigenesis and many other fields. CRISPR technology has been and will be one of the key tools in our understanding and treatment of human cancers.
Reference :
https://www.nature.com/articles/s41568-022-00441-w
CRISPR Applications in Cancer Research Diagnosis and Treatment
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



