2022 Alzheimer’s Disease Global Drug Development Pipeline Summary
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
2022 Alzheimer’s Disease Global Drug Development Pipeline Summary
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
2022 Alzheimer’s Disease Global Drug Development Pipeline Summary.
Is there any special significance to September 1st ? Yes, this day is “World Alzheimer’s Day” .
Everyone is familiar with Alzheimer’s disease (AD), which is also commonly known as “senile dementia”.
Strictly speaking, those with onset before the age of 65 are called Alzheimer’s disease; those with onset after the age of 65 are called Alzheimer’s.
This is a primary degenerative disease of the nervous system , showing a persistent high-level neurological dysfunction, that is, in the absence of disturbance of consciousness, memory, thinking, analysis and judgment, visuospatial recognition, emotion, etc. obstacle.
As the size of the elderly population grows, so does the number of Alzheimer’s disease (AD). There are 9.83 million people with silent disease. There are currently 6.2 million AD patients in the United States, and by 2050 this number will reach 12.7 million.
In addition to AD patients in the dementia stage, there are approximately 10 million people in the United States with mild cognitive impairment (MCI), of which half (5 million) have MCI due to AD.
The global prevalence of AD dementia will increase from the current 50 million to 150 million by 2050, with the majority of those affected living in low- and middle-income countries. The cost of caring for someone with Alzheimer’s disease and related dementia (ADRD) is estimated to be $355 billion in 2021.
Due to the increase in the number of AD patients and the growing public health crisis posed by the disease, the heavy care burden urgently requires treatments to prevent, delay the onset, slow progression, and improve symptoms of AD.
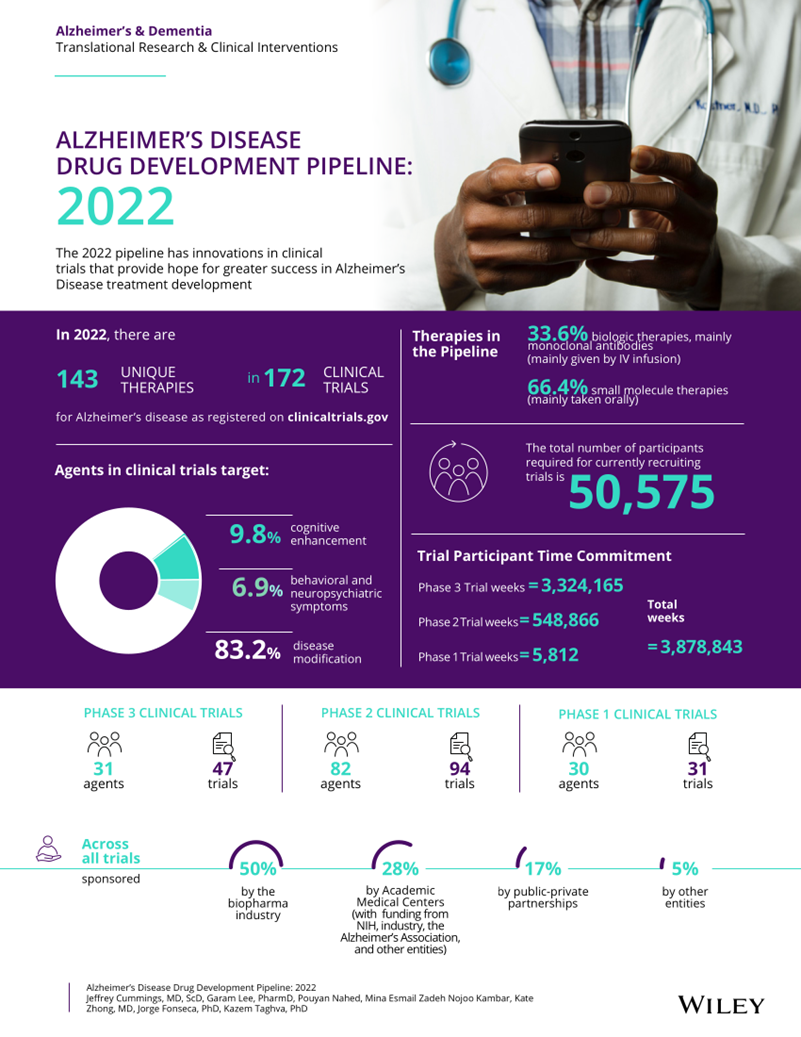
This review , published in March 2022 in Alzheimer’s and Dementia by Jeffrey Cummings et al., reviews current clinical trials and drugs in development for the treatment of AD .
In this review, they used a combination of artificial intelligence (AI) and machine learning (ML) to analyze data from the U.S. National Library of Medicine and clinicaltrials.gov. Contains 172 trials with 143 drugs .
Most of the drugs evaluated are disease-modifying drugs (DMTs) targeting various biological processes involved in AD.
The mechanism of action (MoA) and main trial characteristics of these drugs are summarized.

The update deadline for all data was January 25, 2022, and the analysis included all trials of Phase 1, 2 and 3 drugs.
The mechanism of action (MoA) classification of drugs mainly refers to the classification standards of IADR P (International Alzheimer’s and Related Dementias Research Portfolio) and the National Institute on Aging and the Alzheimer’s Association (National Institute on Aging and the Alzheimer’s Association).
Namely different targets, such as amyloid; Tau; apolipoprotein; lipoprotein receptors; neurotransmitter receptors; neurogenesis; inflammation; oxidative stress; cell death; synaptic plasticity, etc.
Investigators identified 143 drugs in 172 trials in AD (as of January 25, 2022). There are 31 drugs in 47 Phase 3 trials; 82 drugs in 94 Phase 2 trials; and 30 drugs in 31 Phase 1 trials.
Figure 1 shows all AD drugs (both biologics and small molecules) currently in clinical trials. The most common drug under investigation was DMT (119 drugs, or 83.2% of all drugs in trials), disease-modifying therapy; 24 (16.8%) were symptomatic drugs, of which 14 (16.8% of all drugs in trials) 9.8%) were drugs targeting cognitive enhancement, and 10 (6.9% of all trial drugs) were drugs used to treat neuropsychiatric and behavioral symptoms.
Of the DMTs, 40 (33.6% of DMTs) were biologics and 79 (66.4% of DMTs) were small molecules. Twenty (16.8%) DMTs targeted amyloid, 13 (10.9%) targeted tau, 23 (19.3%) targeted inflammation, and 19 (16%) targeted synaptic plasticity/neuroprotection.
Twenty-one (67.8%) Phase 3 drugs were DMT; 71 (86.6%) Phase 2 drugs were DMT; and 27 (90%) Phase 1 drugs were DMT.
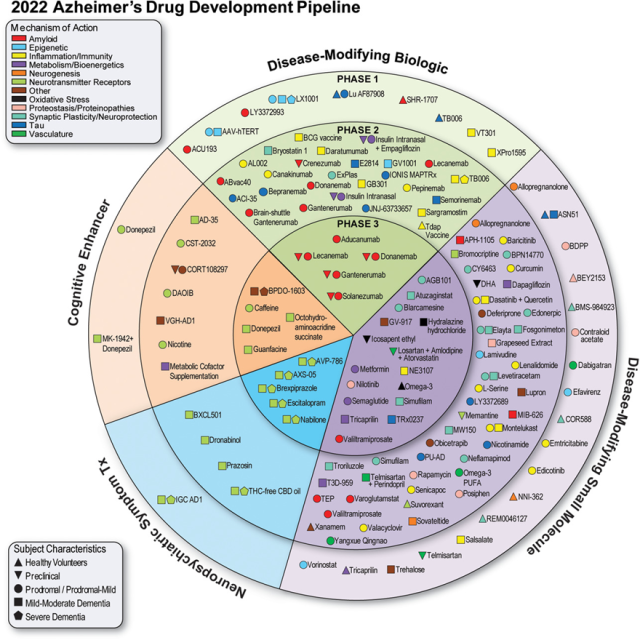
Figure 1 Clinical trial drugs for Alzheimer’s disease in 2021 (from clinicaltrials.gov, deadline January 5, 2021)
(The inner ring is the phase 3 drug; the middle ring is the phase 2 drug; the outer ring is the phase 1 drug; the drugs in the green area are biologics; the drugs in the purple area are small molecules that improve disease; the drugs in the orange area are targeting Medications for cognitive enhancement or behavioral and neuropsychiatric symptoms)
Overview of Phase III Drug Development
There are 31 drugs in 47 Phase 3 trials (Figures 1 and 2, Table 1). Twenty-one (67.8%) drugs in Phase 3 trials were DMTs, including 5 (16.1% of Phase 3 drugs) biologics and 16 (51.6%) small molecule drugs.
Five (16.1% of stage 3 drugs) were cognitive-enhancing drugs and five (16.1%) were for behavioral symptoms.
- CADRO (Common Alzheimer’s Disease Research Ontology) mechanisms in stage 3 DMT include targeting of amyloid (6 drugs; 28.6% of DMTs);
- synaptic plasticity/neuroprotection (4; 19%); oxidative stress (3; 14.3%);
- metabolism and bioenergetics (3; 14.3%); tau (1; 4.8%); inflammation (1; 4.8%);
- proteinosis/proteinopathy (1; 4.8%);
- vasculature (1; 4.8%) ; 4.8%); and the gut-brain axis (1; 4.8%).
Figure 1 shows the MoA of AD stage 3 drugs.
Thirteen of the Phase 3 drugs (42%) were repurposing treatments approved for another indication . In the past year (2021), four Phase 3 drug trials have been completed or terminated.
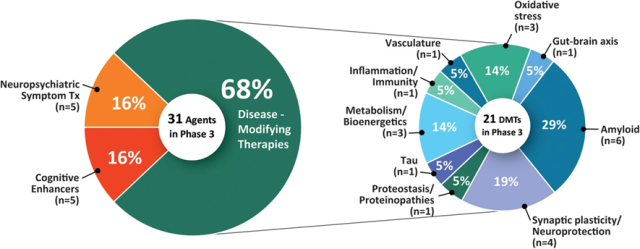 Figure 2 The mechanism of action of the third-stage drug
Figure 2 The mechanism of action of the third-stage drug
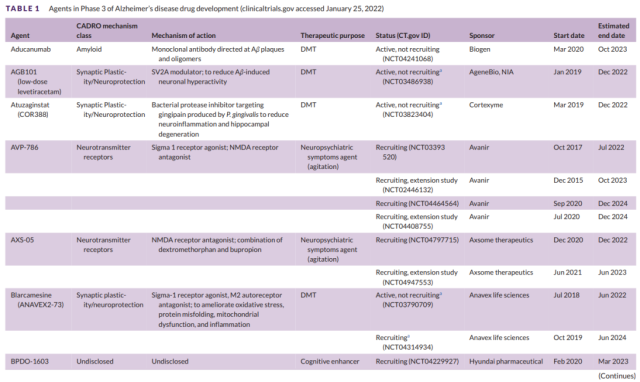 Table 1 Development of Alzheimer’s Disease Phase III Drugs (1)
Table 1 Development of Alzheimer’s Disease Phase III Drugs (1)
 Table 1 Development of Alzheimer’s Disease Phase III Drugs (2)
Table 1 Development of Alzheimer’s Disease Phase III Drugs (2)
 Table 1 Development of Alzheimer’s Disease Phase III Drugs (3)
Table 1 Development of Alzheimer’s Disease Phase III Drugs (3)
 Table 1 Development of Alzheimer’s Disease Phase III Drugs (4)
Table 1 Development of Alzheimer’s Disease Phase III Drugs (4)
Overview of Phase II Drug Development
There were a total of 82 drugs in 94 phase 2 trials (Figures 1 and 3, Table 2). Seventy-one (86.6%) drugs in the phase 2 trials were DMTs, including 26 (31.7% of the phase 2 drugs) biologics and 45 (54.8%) small molecule drugs.
Seven (8.5% of Phase 2 drugs) were cognitive-enhancing drugs and four (4.9%) targeted behavioral symptoms.
- CADRO mechanistic targets in Phase 2 DMT therapy included inflammation (17 drugs; 24% of DMTs);
- synaptic plasticity/neuroprotection (12; 16.9%);
- amyloid (11; 15.5%); tau (9; 12.7%);
- metabolism and bioenergetics (4; 5.6%);
- neurotransmitter receptors (3; 4.2%);
- proteinosis/proteinopathy (3; 4.2%);
- vasculature (3; 4.2%) ; neurogenesis (2; 2.8%);
- growth factors and hormones (2; 2.8%);
- epigenetic regulators (2; 2.8%);
- ApoE, lipid and lipoprotein receptors (1; 1.4%);
- oxidation Stress (1; 1.4%); cell death (1; 1.4%).
Figure 3 shows the mechanism of action of the second stage drug. In the past year (2021), 23 Phase II drug trials have been completed or terminated. There are five trials involving cell therapy in Phase 2 (see Table 4 for details).
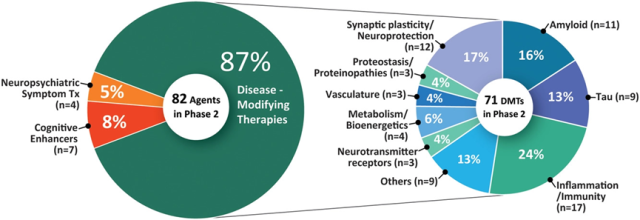 Figure 3 The mechanism of action of the second-stage drug
Figure 3 The mechanism of action of the second-stage drug
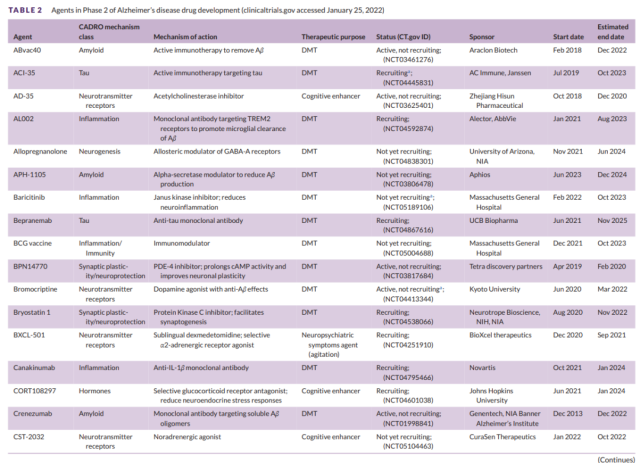 Table 2 Development of second-stage drugs for Alzheimer’s disease (1)
Table 2 Development of second-stage drugs for Alzheimer’s disease (1)
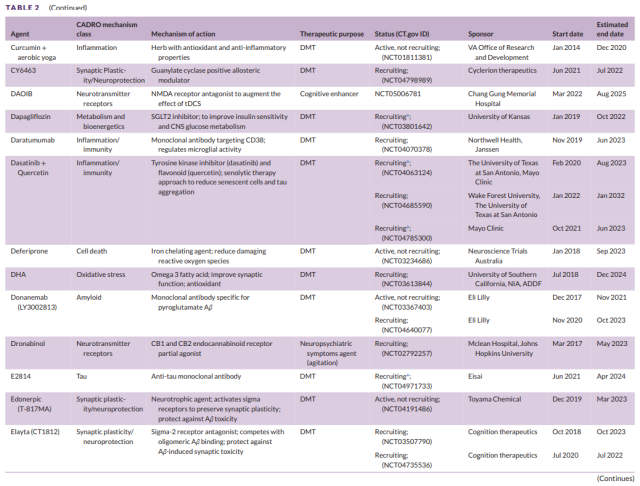 Table 2 Development of second-stage drugs for Alzheimer’s disease (2)
Table 2 Development of second-stage drugs for Alzheimer’s disease (2)
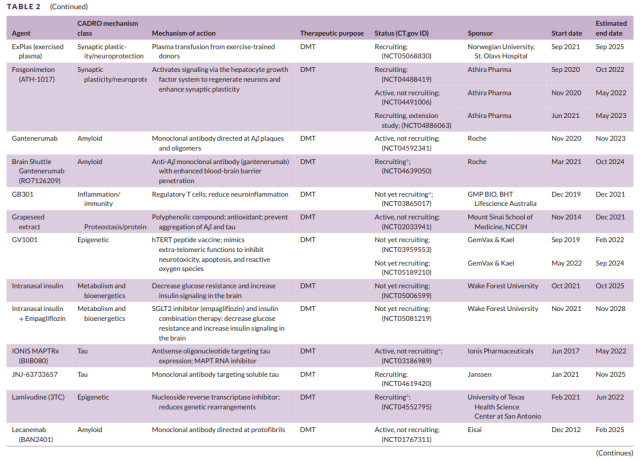
Table 2 Development of second-stage drugs for Alzheimer’s disease (3)
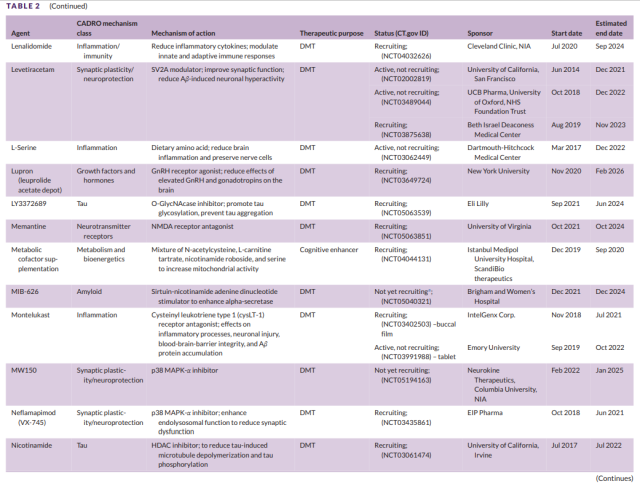 Table 2 Development of second-stage drugs for Alzheimer’s disease (4)
Table 2 Development of second-stage drugs for Alzheimer’s disease (4)
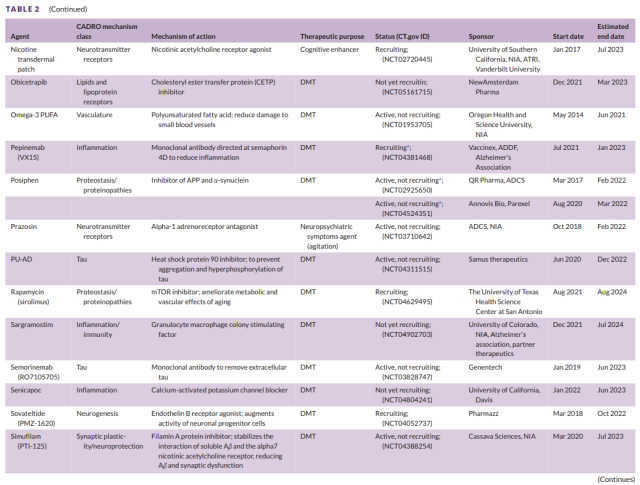 Table 2 Development of second-stage drugs for Alzheimer’s disease (5)
Table 2 Development of second-stage drugs for Alzheimer’s disease (5)
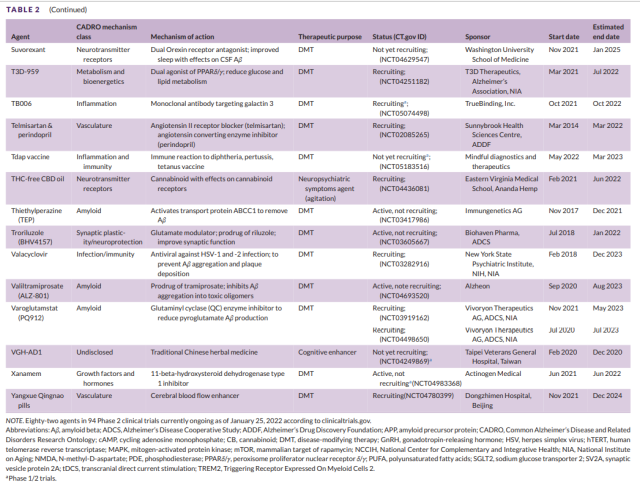 Table 2 Development of second-stage drugs for Alzheimer’s disease (6)
Table 2 Development of second-stage drugs for Alzheimer’s disease (6)
Overview of Phase 1 Drug Development
There were a total of 30 drugs in 31 Phase 1 trials (Figure 1, Table 3). There were 27 DMTs (90% of Phase 1 drugs) in Phase 1 trials, including 9 (30% of Phase 1 drugs) biologics and 18 (60%) of small molecule drugs.
There were two (6.7% of Phase 1 medications) cognitive-enhancing medications and one (3.3%) medications targeting behavioral symptoms. CADRO mechanistic targets in Phase 1 DMT therapy included inflammation (5 drugs; 18.5% of DMTs);
- epigenetic regulators (4; 14.8%);
- amyloid (3; 11.1%);
- tau (3; 11.1%) );
- proteinosis/proteinopathy (3; 11.1%);
- synaptic plasticity/neuroprotection (3; 11.1%);
- neurogenesis (2; 7.4%); vasculature (2; 7.4%);
- cell death ( 1; 3.7%);
- Metabolism and Bioenergetics (1; 3.7%).
Phase 1 has two trials involving stem cell therapy (see Table 4 for details).
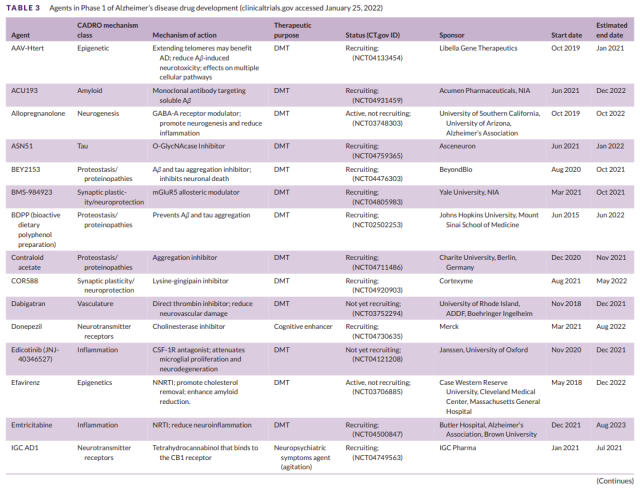
Table 3 Development of Phase I Drugs for Alzheimer’s Disease (1)
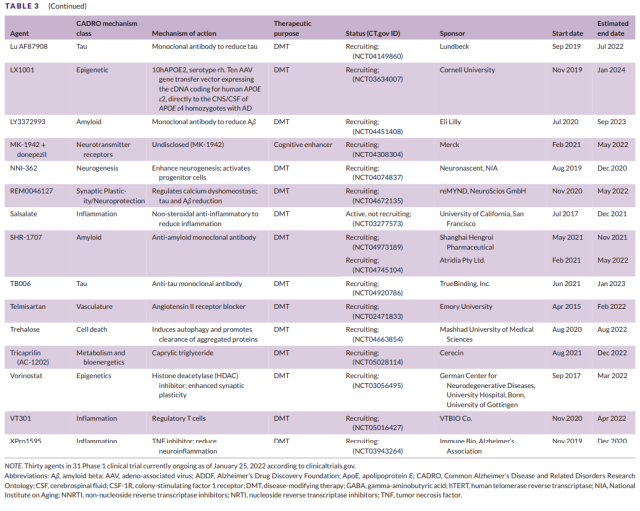 Table 3 Development of Phase I Drugs for Alzheimer’s Disease (2)
Table 3 Development of Phase I Drugs for Alzheimer’s Disease (2)
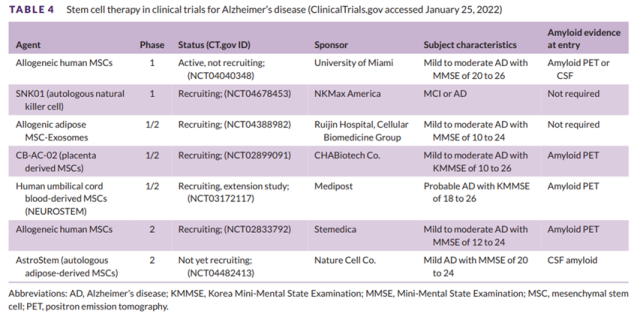
Table 4 Stem cell therapy in Alzheimer’s disease clinical trials
Common biomarkers of Alzheimer’s disease
Table 5 shows the biomarkers used as judgment criteria or outcome measures in DMT’s current Phase 3 and 2 AD clinical trials on clinicaltrials.gov, but may not be complete here, as some trials do not state whether biomarkers are included .
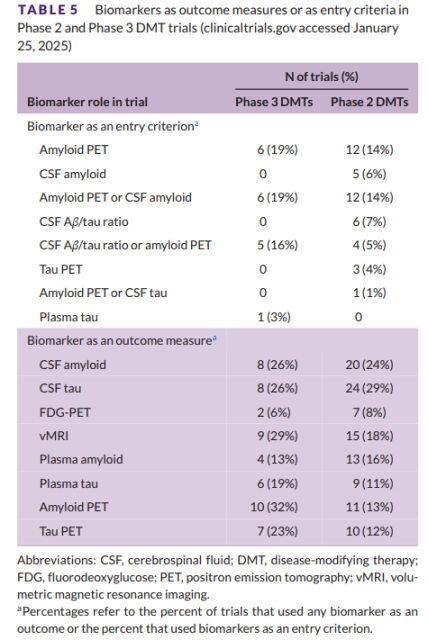 Table 5 Biomarkers used as outcome measures or criteria in phase 2 and 3 DMT trials
Table 5 Biomarkers used as outcome measures or criteria in phase 2 and 3 DMT trials
Of the 31 Phase 3 DMT trials, six trials (19%) used amyloid positron emission tomography (PET) as the criterion and six (19%) used amyloid PET or cerebrospinal fluid (CSF) Amyloid as a measure.
Five of the phase 3 DMT trials (16%) used the CSF-amyloid/tau ratio or amyloid PET as the evaluation criterion, and one trial used plasma phosphorylated tau (p-tau) as the evaluation criterion.
In Phase 2, 12 (14%) DMT trials used amyloid PET as an assessment criterion, 5 (6%) used CSF amyloid or amyloid ratio, and 12 (14%) used amyloid PET or CSF amyloid as assessment criteria.
Six (7%) of the phase 2 DMT trials used the CSF-amyloid/tau ratio as the assessment criterion, 4 (5%) used the CSF-amyloid/tau ratio or amyloid PET, and 3 (4 %) used Tau PET as assessment criteria, and 1 trial (1%) used amyloid PET or CSF-tau as assessment criteria.
Trial Participant Profile
Including all currently ongoing trials, the total number of participants required is 50,575.
Of these, 37,184 participated in the Phase 3 trial; 11,938 participated in the Phase 2 trial; and 1453 participated in the Phase 1 trial.
Table 6 shows the main trial types, the average duration of treatment exposure (in weeks) for each trial, and the number of participants required for each trial.
This indicates that of all ongoing trials, 3,878,843 people will be involved in clinical trials.
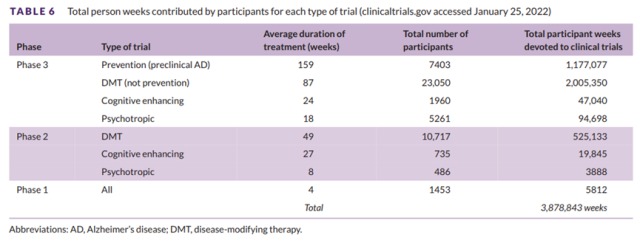 Table 6 Total number of weeks contributed by participants to each trial
Table 6 Total number of weeks contributed by participants to each trial
Test applicant
Of all trials in AD drug development , 50% are funded by the biopharmaceutical industry, 28% are funded by academic medical centers (often NIH-funded), 17% are funded by public-private partnerships, and 5% are funded by other entities.
In Phase 3, 68% of trials were sponsored by the biopharmaceutical industry, 15% by academic medical centers/NIH, 11% by public-private partnerships, and 6% by others.
In Phase 2, 41% of trials were sponsored by the biopharmaceutical industry, 36% were sponsored by academic medical centers/NIH, 19% were public-private partnerships, and 3% were funded by others. Table 7 shows the sponsors for each development stage.
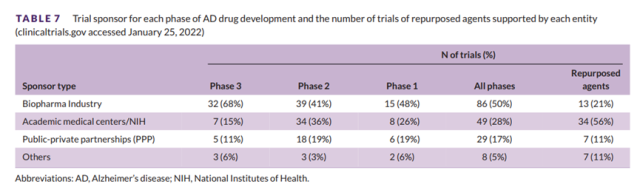 Table 7 Trial sponsors per stage of AD drug development and number of repurposing drug trials supported by each entity
Table 7 Trial sponsors per stage of AD drug development and number of repurposing drug trials supported by each entity
Global distribution of AD drug trials
Table 8 summarizes the global distribution of AD drug trials. Thirty-six percent of Phase 3 trials involved North America only, and 40% included both North American and non-North American countries. 54% of Phase 2 trials were typically conducted in North America; 64% of trials listed North America as a trial site (trials conducted in North America only plus trials conducted at North American and non-North American sites). Across the three phases, 48% of trials were conducted in North America only; 33% were conducted outside North America only; and 20% were conducted in North America and non-North American regions. North America participated in 67% of all trials registered on clinicaltrials.gov.
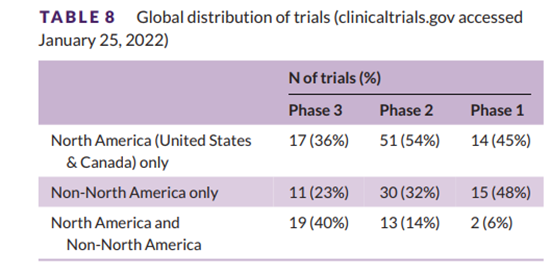 Table 8 Global test distribution
Table 8 Global test distribution
Summary: 2022 Alzheimer’s Disease Global Drug Development Pipeline Summary
Figure 4 summarizes the mechanism of action of disease modifiers in all phases of clinical trials classified by CADRO target.
These include drugs targeting amyloid (20), tau (13), inflammation (23) and synaptic plasticity (19). Of the 17 categories (excluding unknown and other categories), a total of 15 categories are drugs in development.
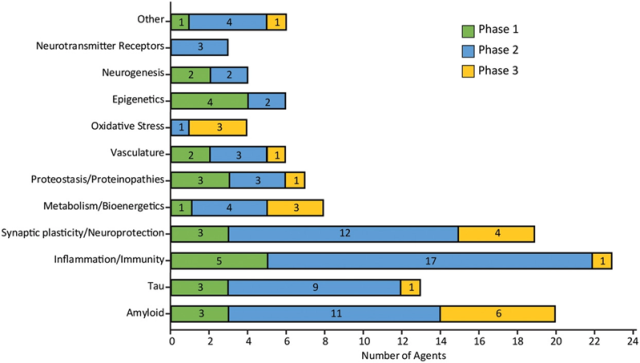
Figure 4 Mechanisms of action of disease modifiers in all phases of clinical trials grouped by common CADRO
Clinical trials in AD target a robust range of biological processes, including most of those identified in the CADRO classification.
Amyloid therapy has come a long way with the approval of the monoclonal antibody aducanumab. Other approaches targeting amyloid and treatments for tau abnormalities, inflammation, and synaptic dysfunction are well represented in the AD drug development pipeline.
Despite the challenges posed by the current coronavirus pandemic, clinical trials of AD drugs are still ongoing. Biomarkers are increasingly being used to inform clinical trials, including their use in diagnosis and outcomes. New clinical outcome measures, especially composite scales and scores, provide increased sensitivity to treatment response.
Drug development for AD depends on strong alliances with participants and their families as they make huge time sacrifices in trial participation.
The distribution of trial sites showcases the global ecosystem that supports AD drug development.
Advances in target identification, drug discovery, and clinical trial approaches have increased confidence that more and better treatments will emerge in the AD drug development pipeline.
References:
https://alz-journals.onlinelibrary.wiley.com/doi/10.1002/trc2.12295
https://www.thelancet.com/journals/lanpub/article/PIIS2468-2667(20)30185-7/fulltext
Alzheimer’s Disease – A+ Medical Encyclopedia (a-hospital.com)
2022 Alzheimer’s Disease Global Drug Development Pipeline Summary
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



