This type of diet can trigger cancer cells to self-destruct
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
This type of diet can trigger cancer cells to self-destruct
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
This type of diet can trigger cancer cells to self-destruct.
Ferroptosis is a novel form of programmed cell death that is iron-dependent and distinct from apoptosis, necrosis, and autophagy.
It is caused by the accumulation of iron-dependent lipid peroxides and was first proposed by Professor Brent R. Stockwell from Columbia University in 2012.
The main mechanism of ferroptosis involves the catalysis of highly expressed unsaturated fatty acids on the cell membrane by divalent iron or lipoxygenase, leading to lipid peroxidation and subsequent induction of cell death.
Additionally, it is characterized by the downregulation of the core enzyme GPX4, a key regulator in the antioxidant system (glutathione system).
The main mechanisms of death are:
GPX4 inactivation based on GSH depletion:
GPX4 inactivation:
Iron ion input and iron ion reduction:
Glutathione plays a crucial role in the process of ferroptosis, as it is an antioxidant in the human body that inhibits the activity of glutathione peroxidase 4 (GPX-4), thereby suppressing lipid peroxidation and inhibiting the process of iron-dependent cell death.
Glutathione is directly synthesized from the sulfur-containing amino acid cysteine; therefore, restricting the intake of cysteine and methionine through diet (cysteine and methionine deprivation, CMD) can limit the generation of glutathione in the body and promote iron-dependent cell death in cancer cells, effectively controlling tumor growth.
A recent study published in Nature Communications focused on glioblastoma, a malignant tumor originating in the brain.
The research demonstrated that controlling tumor growth can be achieved by restricting the intake of cysteine and methionine, as treatment resistance and oxidative stress in this tumor type are closely associated with the metabolism and oxidative stress of glutathione.
CMD induces ferroptosis in glioma cells
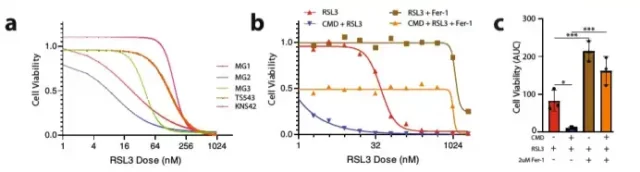
Changes of CMD on metabolic function in glioma mice
The effect of CMD on the survival rate of glioma mice
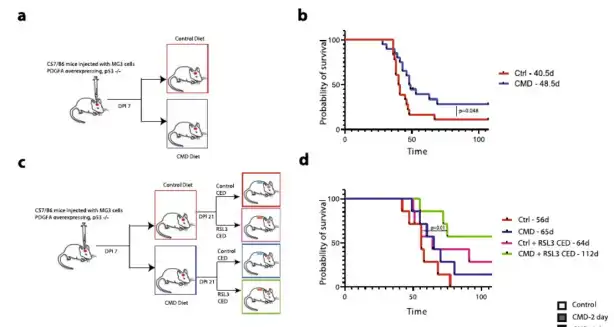
Animal experiments on mice were first divided into two groups. Seven days after the injection of MG3 glioma cells, the mice were fed with the control diet (0.43% w/w methionine, 0.40% w/w cystine) and CMD respectively.
Diet (0.15% w/w methionine, 0% w/w cystine), the results of the study found that although the diet of CMD had a significant effect on the weight loss of mice, the survival rate (OS) of CMD mice was significantly better than in the control group (above b).
The synergy of CMD diet with RSL3 treatment was next tested by dividing mice into 4 groups: control diet (0.43% w/w methionine, 0.40% w/w cystine) ± RSL3 treatment and CMD diet (0.15% w/w /w methionine, 0% w/w cystine)±RSL3 treatment, the study found that the CMD+RSL3 treatment group had the longest survival time and was significantly better than the control group, followed by the RSL3 treatment group, followed by the CMD diet, and the control group The diet group had the worst survival (c and d above panel).
Continued analysis of changes in the lipidome, metabolome, and proteome of the experimental mice, including acetylmethionine, adenosylhomocysteine, cysteine, oxidized glutathione, and formazan after the CMD diet Concentrations of several metabolites including thionine were reduced in the tumor volume.
Methionine showed a downward trend on the 2nd day, and decreased significantly on the 4th day, while oxidized glutathione showed a decrease on the 2nd day of CMD, but this difference narrowed on the 4th day, which suggested Compensatory mechanisms may exist in mice.
Metabolite analysis of the CMD diet or the control diet showed decreased levels of hypotaurine and oxidized/total glutathione, as well as decreased levels of cysteine. Quantitative metabolite pathway analysis revealed significant alterations (p < 0.10) in taurine/hypotaurine metabolism, glutathione metabolism, arginine metabolism, tricarboxylic acid cycle, and fatty acid elongation/degradation.
Proteomic analysis found that other genes/proteomes were enriched in the CMD group compared with the control group and affected immunosuppression.
In vitro medium and mouse experiments confirmed that restriction of cysteine and methionine intake (cysteine and methionine deprivation CMD) exerted anti-tumor effect through ferroptosis mechanism, and CMD affected the activity of glioma and Prolonging the survival of glioma mice, this diet can also enhance the anti-tumor effect of GPX-4 inhibitors.
However, the CMD diet suppressed immunity and had a significant effect on weight loss in mice, so its safety needs to be further explored.
This type of diet can trigger cancer cells to self-destruct.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



