Why did Japanese vaccine companies response so slow during COVID pandemic?
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Why did Japanese vaccine companies response so slow during COVID pandemic?
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Why did Japanese vaccine companies response so slow during COVID pandemic?
On February 17, 2021, Dr. Kazuhiro Araki, the director of Tokyo Medical Center, received the first dose of the Pfizer-BioNTech vaccine, marking the start of COVID-19 vaccination in Japan.
According to a report by the Japan Broadcasting Corporation on May 11 of the same year, the percentage of the population that had received their first dose of the COVID-19 vaccine was 63% in Israel, 52% in the UK, 46% in the US, but only 2.91% in Japan.
Despite the Tokyo Olympics being less than 60 days away, Japan’s COVID-19 situation showed no signs of improvement. Japanese media continuously questioned issues like “disorganized vaccine administration” and “difficulty in obtaining vaccine doses.”
Many people wondered why Japan, as the world’s third-largest pharmaceutical market, with multinational giants like Astellas, Takeda, and Daiichi Sankyo, was lagging behind in vaccine production.
The reason lies in the lack of domestically developed vaccines. In mid-April, Japanese Prime Minister Yoshihide Suga had to personally call the CEO of Pfizer, hoping to secure additional vaccine supplies.

As for Japan’s domestically-produced vaccine, it arrived too late. On July 31, 2023, Daiichi Sankyo’s mRNA COVID-19 vaccine targeting the original strain was approved and became Japan’s first domestically produced COVID-19 vaccine to receive approval.
However, by now, the pandemic has gradually subsided, and the COVID-19 strains have evolved into new generations, rendering vaccines targeting the original strain outdated. For Japan, the symbolic significance of this vaccine outweighs its practical value.
The weakness of Japan’s vaccine industry is evident. In fact, several decades ago, Japan’s vaccine industry was not only competitive but also considered among the best globally.
However, with a series of vaccine safety issues emerging, public confidence in vaccines continuously diminished, making Japan one of the countries with the lowest vaccine confidence worldwide. Consequently, the vaccine market shrank, and the enthusiasm of companies for developing new vaccines plummeted.
Since the 1980s, Japan’s vaccine industry has been experiencing severe decline, leading to a 15-year hiatus. It wasn’t until the COVID-19 outbreak that Japan found itself in a situation where vaccines were scarce and difficult to obtain.
In any industry, at any time, the most dreadful thing is not technological barriers or fierce competition but the loss of confidence in the future development for everyone involved.
Escalating Trust Crisis
Taking a trip back to the 1980s, Japan’s vaccine technology was at the forefront globally, particularly with top-notch vaccines for diseases like chickenpox, Japanese encephalitis, and pertussis, which were even authorized for use in the United States and other regions.
So, how did Japan, holding such a winning hand, end up losing?
This traces back to a series of vaccine scandals that occurred decades ago. In 1975, two deaths were reported in Japan following the administration of the DTwP vaccine, leading to a temporary suspension of its use. This incident sparked skepticism among the Japanese population towards vaccination.
While the public’s doubts about vaccines were still simmering, another issue arose with mandatory vaccines in Japan. In 1989, an introduced measles-mumps-rubella (MMR) combination vaccine resulted in over 1,700 cases of aseptic meningitis among 1.8 million vaccinated newborns.
Interestingly, there were vaccines available overseas with even lower rates of aseptic meningitis, but to protect domestic vaccine companies, the Japanese government delayed the adoption of these safer vaccines, exacerbating the public’s mistrust of vaccines.
Subsequently, Japan witnessed several other vaccine safety incidents. In the 1980s, the “HIV-tainted blood products” issue affected 1,800 hemophilia patients who were infected with HIV due to contaminated blood products. In 2015, Kaketsuken, a center for vaccines and blood products in Japan, was exposed for decades-long fabrication of data.
All these scandals further deepened the Japanese population’s lack of trust in vaccines.
According to a study published in The Lancet, Japan is one of the countries with the lowest confidence in vaccines, with less than 30% of people strongly believing that vaccines are safe, important, and effective, while the corresponding figure is at least 50% in the United States.
The loss of trust and negative public opinion about vaccines have had consequences, as seen in the notorious HPV vaccine controversy in Japan.
The HPV vaccine, now widely administered globally, was developed by a Japanese company over a decade ago. In 2010, the Japanese Ministry of Health, Labour, and Welfare included the HPV vaccine in the national immunization program. However, many girls who received the vaccine experienced symptoms such as fainting and chronic pain. Affected individuals in various regions demanded accountability from pharmaceutical companies and the Japanese government, believing that the government approved and recommended the vaccine without ensuring its safety.
There is a saying in Japan, “Safety is free.” While most people may tolerate side effects during treatment, they cannot accept harm for the sake of disease prevention. Over the years, many Japanese media outlets have consistently questioned the efficacy of the HPV vaccine and repeatedly reported potential adverse reactions following its administration.
Under the pressure of public opinion, the Japanese government withdrew its recommendation for this vaccine. By 2020, the HPV vaccine coverage rate in Japan had dropped to 0.1%, compared to 80% in the United States.
This has significantly dampened enthusiasm and motivation for vaccine development among companies in Japan.
The lost of fifteen Years: Japan’s Vaccine Industry in Crisis
Faced with a crisis of public trust in vaccines and growing public condemnation, a series of ill-advised actions by the Japanese government has not only failed to salvage the country’s vaccine industry but has plunged it into a deep abyss.
Following the infamous MMR vaccine incident mentioned earlier, the Japanese government was taken to court.
In 1992, the Tokyo High Court ruled that the government should be held responsible for adverse reactions, including side effects, to multiple vaccines even in the absence of scientific evidence linking the vaccines to these events. As a result, the government had to pay a staggering 3.2 trillion yen in settlements to handle the litigations.
In theory, to reduce the occurrence of similar adverse events, the Japanese government should have invested more manpower, resources, and funding to guide the industry in developing vaccines with fewer side effects and better efficacy.
However, in reality, a sense of “we will be blamed whenever a vaccine issue arises” permeated the Japanese government after the ruling. Dealing with vaccine-related problems, the government began to choose the path of shifting blame.
In 1994, the Japanese government amended the “Vaccination Law,” changing the “mandatory vaccination” to “voluntary efforts.” With this subtle difference, the fate of Japan’s vaccine industry was drastically altered.
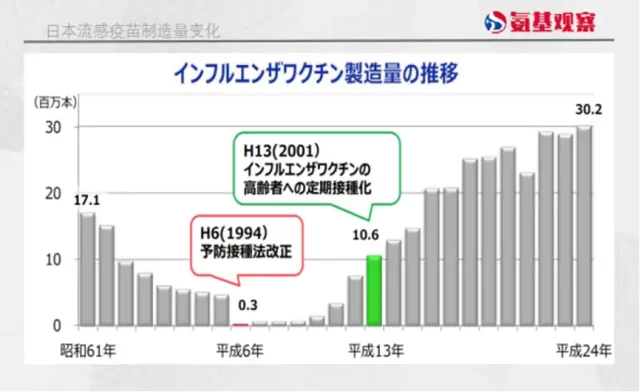
Already lacking trust in vaccines, the Japanese public saw vaccination becoming voluntary, resulting in a significant decline in vaccination rates. For instance, flu vaccination within Japan nearly disappeared starting from 1994.
Simultaneously, as vaccination rates continued to decline, the vaccine market shrank in proportion to the decrease in the child population, leading to a contraction in Japan’s vaccine market size.
Furthermore, the Japanese government began to tighten vaccine approvals. From 1993 to 2007, not only did Japan’s vaccine development stagnate, but it also imported only two new vaccines from overseas, demonstrating an excess of caution, while the United States introduced 17 new vaccines during the same period.
This created a fifteen-year gap in Japan’s vaccine development, gradually pushing the country’s vaccine industry into stagnation and even decline.
Unfriendly Regulatory Environment
Over the past decade, Japan’s policies and regulatory environment have dealt a blow to the vaccine industry.
On one hand, the “protective measures” imposed by the Ministry of Health, Labour, and Welfare have hindered the growth of Japan’s vaccine companies. This “protective measures” policy is aimed at safeguarding specific industries, especially those with weaker competitiveness like small and medium-sized enterprises, by fostering and developing them through strong administrative leadership and closed industry systems. Under this system, established vaccine manufacturers producing conventional vaccines can thrive, while those working on the development of new vaccines receive little government assistance. A prime example of this issue is the bankruptcy of the Japanese biopharmaceutical company UMN Pharma.
UMN Pharma attempted to develop a genetically recombinant flu vaccine using cutting-edge technology and invested over 10 billion yen in constructing a factory. However, due to lack of approval, UMN Pharma withdrew its application in 2017. Subsequently, UMN Pharma faced financial difficulties and was acquired by Yamanouchi Pharmaceutical Co., Ltd.
On the other hand, the Japanese government has consistently failed to provide sufficient financial support for research and vaccine development. In 2015, during the Middle East Respiratory Syndrome (MERS) outbreak in South Korea, the Japanese government commissioned Professor Ken Ishii to work on an mRNA vaccine for MERS. However, when the research team successfully completed animal experiments, the government halted further funding, citing the end of the epidemic. As a result, the development of the mRNA vaccine was abandoned. If the Japanese government had continued to invest in mRNA vaccine technology, they might not have faced the current situation of relying solely on foreign vaccines during the COVID-19 pandemic.
Even during the pandemic, Japan’s budget allocation for COVID-19 vaccine research remained inadequate. In May 2020, the Japanese government allocated approximately 200 billion yen (around 1.85 billion USD) for COVID-19 vaccine research and production. In contrast, the United States launched the “Operation Warp Speed” initiative, providing 18 billion USD to accelerate COVID-19 vaccine, drug development, and testing.
Without proper comparison, one cannot fully grasp the extent of the damage. Vaccine development is a time-consuming, labor-intensive, and expensive process. However, the Japanese government’s reluctance to allocate sufficient funds and lack of proactive policy guidance has almost sealed the fate of the declining vaccine industry in Japan.
Reviving Confidence in Vaccine Industry
Japan faces two formidable challenges in the vaccine innovation landscape – an unfriendly public opinion and legal environment. Questions like “Why is domestically produced vaccine delayed in medically advanced Japan?” and “When will Japan achieve nationwide COVID-19 vaccine coverage?” persistently plague the government, with dissatisfaction rates among the public exceeding 60%.
The COVID-19 pandemic has made the Japanese government acutely aware of the importance of vaccines for the nation. Kengo Okada, the Chairman of the Japan Vaccine Society, emphasized in an interview with the local media that the development of COVID-19 vaccines requires collaboration between industry and academia, going beyond being merely a healthcare policy under the Ministry of Health, Labor, and Welfare. It should be an integral part of national crisis management.
Now, the Japanese government is determined to catch up in the field of vaccines. In March 2022, they established the Advanced Biomedical Vaccine Research and Development Preparation and Response Strategy Center to address issues such as manpower shortage, financial constraints, and slow regulatory processes hampering vaccine research in Japan.
Furthermore, Japan is pushing forward a 1.1 trillion yen (85 billion USD) investment plan to enable the country to develop vaccines against new viruses within 100 days.
However, whether these delayed policies and investments, after fifteen years of stagnation, can bring the Japanese vaccine industry back on track remains uncertain. Concerns about vaccine safety among the Japanese public still persist, preventing a swift recovery of the vaccine market. These issues cannot be resolved merely through funding or administrative orders.
Reflecting on the decline of Japan’s vaccine industry, it becomes evident that the government failed to restore the lost public confidence after safety issues arose, instead opting to avoid responsibility repeatedly. Consequently, the choices made by the Japanese government sowed the seeds of a vaccine shortage when facing the COVID-19 pandemic.
The decline of Japan’s vaccine industry serves as a profound warning, emphasizing that the trajectory of an industry is not determined by unpredictable administrative orders but rather by the expectations and confidence of its stakeholders. This, in turn, shapes the industry’s future.
Once precious confidence is lost, catching up subsequently may require manifold investments.
Why did Japanese vaccine companies response so slow during COVID pandemic?
(source:internetHBjylDHPi4f61IPcuV6oOQ, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



