Why Are Humans Prone to Alzheimer’s Disease?
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Why Are Humans Prone to Alzheimer’s Disease? Long Non-Coding RNA Implicated
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Science: Why Are Humans Prone to Alzheimer’s Disease? Long Non-Coding RNA Implicated.
Alzheimer’s disease (AD), often referred to as “senile dementia,” stands as the most prevalent neurodegenerative disorder. AD patients typically exhibit symptoms such as memory decline, reduced learning abilities, emotional regulation disturbances, and loss of motor skills, rendering them heavily dependent and placing a substantial burden on families and society.
In some ways, Alzheimer’s disease can be even more daunting than cancer. Its etiology is multifaceted, and effective therapeutic drugs have long been elusive, with clinical treatments often limited to symptom alleviation. What’s even more alarming is the escalating incidence of Alzheimer’s disease alongside the global aging trend, with an estimated 150 million patients projected by 2050. Consequently, Alzheimer’s disease has earned the moniker of the “21st-century pandemic.”
Neuronal loss represents a pivotal hallmark of Alzheimer’s disease, yet its underlying mechanisms remain obscure.
On September 14, 2023, the team led by Bart De Strooper at the VIB-KU Leuven Center for Brain and Disease Research in Belgium published a research paper titled “MEG3 activates necroptosis in human neuron xenografts modeling Alzheimer’s disease” in the prestigious academic journal Science.
The study indicates that in the brains of Alzheimer’s disease mouse models transplanted with human or murine neurons, only human neurons exhibit severe Alzheimer’s disease pathology, including neurofibrillary tangles and necrotic apoptosis. Long non-coding RNA MEG3 is markedly upregulated in the Alzheimer’s disease-affected human neurons, and pharmacological or genetic reduction of MEG3 rescues neuronal loss in xenografted human neurons.
This discovery unveils the unique susceptibility of human neurons to Alzheimer’s disease and hints at potential therapeutic targets for the condition.

Beta-amyloid (Aβ) plaques, neurofibrillary tangles, granulovacuolar degeneration (GVD), and neuronal loss are common pathological features of Alzheimer’s disease. Alzheimer’s disease mouse models, although classic for research, are often artificially induced and fail to elucidate the interplay of these pathological features.
In fact, fundamental questions regarding whether Aβ pathology can induce tau tangles and how neurons die in Alzheimer’s disease have remained unanswered. Two excellent models for replicating human pathological features of Alzheimer’s disease include 3D human brain organoids and xenografted human neurons in the mouse brain.
In this latest study, the research team improved the xenograft model on Nod-SCID mice, incorporating immunosuppressive genetic backgrounds with a single AppNL-G-F gene insertion to drive Aβ pathology. They transplanted human-derived neural progenitor cells (NPCs) into the brains of these Aβ-bearing mice, which integrated well and developed neuronal dendritic spines. Two months post-transplantation, the xenografted neurons already exhibited characteristics of mature neuronal markers (NEUN, MAP2) and cortical markers (CTIP2, SATB2, TBR1, CUX2).
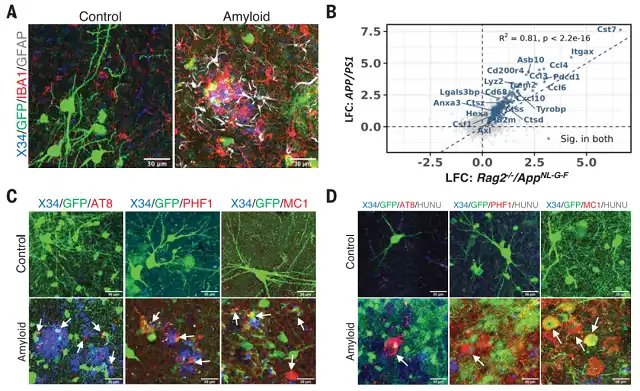
Deposition of amyloid plaques is sufficient to induce tangles in xenografted animal models
In comparison to the control group with transplanted mouse neurons, mice with transplanted human neurons displayed severe Alzheimer’s disease pathology, including neurofibrillary tangles, granulovacuolar degeneration (GVD), phosphorylated tau protein blood biomarkers, and substantial neuronal loss. This suggests the presence of unknown human-specific features that define the sensitivity of human neurons to Aβ pathology.
Xenografted human neurons exhibit severe Alzheimer’s disease pathology
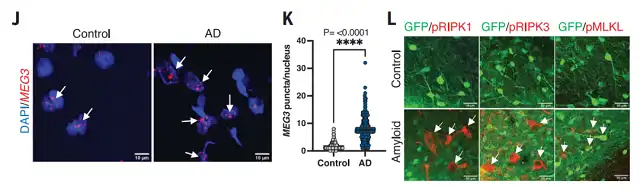
Transcriptomic analysis revealed a significant upregulation, approximately tenfold, of a long non-coding RNA (lncRNA) known as Neuron-Specific Maternally Expressed Gene 3 (MEG3) in the affected human neurons. It’s worth noting that this neuron-specific long non-coding RNA also showed a 2-3 fold upregulation in Alzheimer’s disease patients.
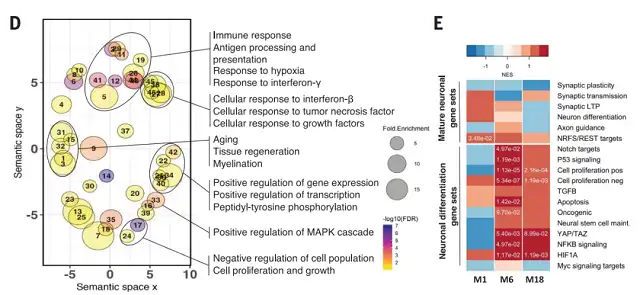
Transcriptomic changes in xenografted human neurons
Further research indicated that MEG3 overexpression in vitro was sufficient to induce necrotic apoptosis in human neurons. In contrast, downregulating MEG3 through pharmacological or genetic manipulation of receptor-interacting protein kinases (RIPK1, RIPK3) or mixed lineage kinase domain-like protein (MLKL) inhibited necrotic apoptosis in human neurons, rescuing neuronal cell loss in the xenograft animal model.
Image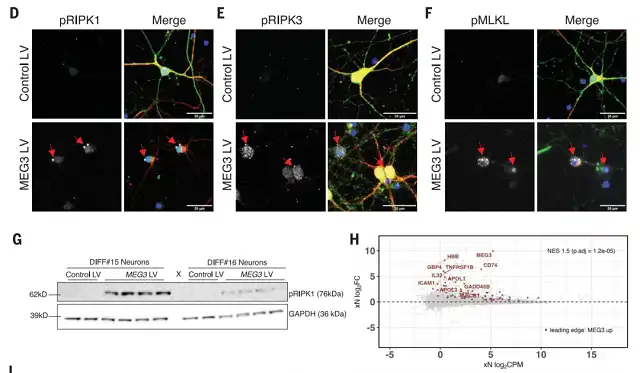 NA-MEG3 induces necrotic apoptosis in human neurons
NA-MEG3 induces necrotic apoptosis in human neurons
In summary, this latest research published in Science demonstrates that Aβ pathology can induce necrotic apoptosis in human neurons, with MEG3’s sharp upregulation playing a crucial role.
Consequently, neuronal loss in Alzheimer’s disease can potentially be rescued by downregulating MEG3 expression or inhibiting the necrotic apoptosis pathway, opening up novel potential targets and approaches for Alzheimer’s disease prevention and treatment.
Science: Why Are Humans Prone to Alzheimer’s Disease? Long Non-Coding RNA Implicated
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



