FDA issued guidelines for bispecific antibody development
- Why Lecanemab’s Adoption Faces an Uphill Battle in US?
- Yogurt and High LDL Cholesterol: Can You Still Enjoy It?
- WHO Releases Global Influenza Vaccine Market Study in 2024
- HIV Infections Linked to Unlicensed Spa’s Vampire Facial Treatments
- A Single US$2.15-Million Injection to Block 90% of Cancer Cell Formation
- WIV: Prevention of New Disease X and Investigation of the Origin of COVID-19
FDA issued guidelines for bispecific antibody development
FDA issued guidelines for bispecific antibody development. FDA issued the guidelines for the finalization of the Bispecific Antibody Development Project on May 24, providing advice to the industry and parties involved in the development of bispecific antibodies, including general supervision and scientific considerations for bispecific antibodies.
The guidelines point out It addresses the challenges that may arise in the development of bispecific antibodies and provides recommendations on the type of data required to support approval.
Bispecific antibodies are currently the hotspot of clinical development at home and abroad. They are composed of two different binding domains and can bind to two different antigens or two different determinants of the same antigen. According to the mechanism of action, bispecific antibodies can be roughly divided into two types:
(1) Immune cell-oriented bispecific antibodies can bridge two target cells (for example, a bispecific antibody designed to bring immune effector cells into close contact with specific tumor-associated antigens to promote cell killing). This type of bispecific antibody needs to bind to two targets and be effective at the same time.
(2) Have bispecific antibodies that do not involve bridging two target cells, such as bispecific antibodies against two soluble cytokines, which bind to different epitopes of the same tumor cell or viral antigen, or with two different targets Combine to mimic functional endogenous protein. In this category, bispecific antibodies sometimes (but not always) need to bind to two targets at the same time in order to be effective.
Bispecific antibodies have potential advantages over other therapies. One drug can be used to target multiple disease-regulating molecules, which has become a hot spot in the field of antibody engineering. The FDA stated that the guidance clarifies the quality, non-clinical, and clinical considerations when developing bispecific antibodies. The guidelines do not include guidance on the regulatory pathways for evaluating bispecific antibodies.
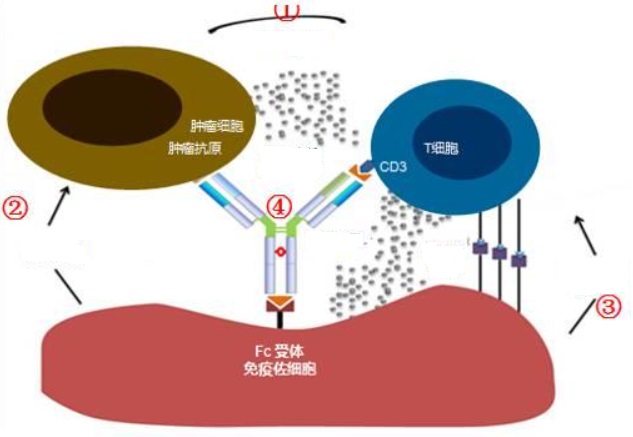
Schematic diagram of the mechanism of action of bispecific antibodies, from the Internet
Currently, three bispecific antibodies have been approved by the FDA for marketing:
1. Blincyto (blinatumomab) developed by Amgen, which targets CD3 and CD19 at the same time, is a T cell-directed bispecific antibody for minimal residual disease (MRD) positive precursor B-cell acute lymphoblastic leukemia (pre-B ALL) Treatment of adult and pediatric patients;
2. Roche’s Hemlibra (emicizumab-kxwh) is a bispecific antibody that can simultaneously bind FIX and FX. By bridging the activated FIX and FX, it restores the function of the missing coagulation factor VIII, thereby effectively stopping bleeding and preventing hemophilia. Bleeding of the sick patient;
3. Johnson & Johnson’s Rybrevant (amivantamab-vmjw) is a bispecific antibody that simultaneously targets EGFR and cMET. It can not only block the binding of ligands to EGFR and MET, promote receptor degradation, but also trigger antibody dependence. Sexual cell cytotoxicity is the first therapeutic agent for adult patients with non-small cell lung cancer with an insertion mutation in exon 20 of the epidermal growth factor receptor (EGFR).
What is revised majorly?
The guidelines finalized the draft guidelines of the same title issued on April 19, 2019. The FDA considered feedback from the industry on the draft guidelines when finalizing the guidelines. The main changes to the draft guidelines include:
- Focus on the quality of bispecific antibodies, the unique aspects of non-clinical and clinical development plans;
- Clarify the potential immunogenicity associated with bispecific antibodies;
- To clarify the clinical evaluation of comparing bispecific antibodies and approved monospecific antibody products.
After the release of the 2019 draft guidance, many pharmaceutical manufacturers commented on the FDA’s statement in the draft guidance that “there are many challenges in the development of bispecific antibodies, one of which may be the significant immunogenicity caused by neoantigenic determinants” Expressed strong opposition. Therefore, the FDA changed this sentence in the finalized guidelines to “the development of bispecific antibodies may encounter many challenges, such as immunogenicity related to novel structures and complex structural and functional characterization.”
Regarding the mechanism of action of specific double antibodies, the guidelines clarify some of the issues to be studied, including determining whether two targets need to be combined at the same time, and the affinity, binding/dissociation rate and potential synergistic effects of each target need to be meticulous the study. The guidelines emphasize scientific development, including targets, mechanism of action, safety, and effectiveness relative to single-target drugs, which need to be considered.
In terms of CMC, the characteristics of the product should be determined according to standard monoclonal antibody development practices, and the manufacturing process should be developed. Some quality factors that may directly affect the mechanism of action need to be considered. For example, the double antibody binds to two target antigens on the same cell at the same time.
In terms of clinical research, the guidelines emphasize that if there are monospecific antibody products approved for the same indication and they are the same as the target antigen of the bispecific antibody, a clinical comparison between the bispecific antibody and the monospecific product is necessary if necessary Research to conduct benefit-risk assessments more effectively.
(source:internet, reference only)
Disclaimer of medicaltrend.org



