NEJM: One shot in two months to achieve long-term prevention of AIDS
- A Single US$2.15-Million Injection to Block 90% of Cancer Cell Formation
- WIV: Prevention of New Disease X and Investigation of the Origin of COVID-19
- Why Botulinum Toxin Reigns as One of the Deadliest Poisons?
- FDA Approves Pfizer’s One-Time Gene Therapy for Hemophilia B: $3.5 Million per Dose
- Aspirin: Study Finds Greater Benefits for These Colorectal Cancer Patients
- Cancer Can Occur Without Genetic Mutations?
NEJM: One shot in two months to achieve long-term prevention of AIDS
NEJM: One shot in two months to achieve long-term prevention of AIDS. Despite effective strategies to prevent human immunodeficiency virus (HIV) infection, the number of new infections worldwide still exceeds 5,000 every day. Previous studies have shown that daily oral tenofovir disoproxil-emtricitabine fumarate (TDF-FTC)) can provide protection against HIV infection in different populations.
The efficacy of oral pre-exposure prophylaxis (PrEP) drugs is directly related to compliance with prescribed doses. PrEP drugs that do not require regular or planned oral administration may increase acceptability and protection during the risk period, thereby reducing the risk of HIV infection.
Therefore, there is a need for safe and effective long-acting drugs for pre-exposure prevention (PrEP) of HIV infection to increase the options for preventing HIV infection.
Long-acting injectable Cabotegravir (CAB-LA) is an integrase inhibitor that can inhibit the replication process of retrovirus and block the integration of retrovirus and host chromosome. Previous studies have shown that Cabotegravir has strong anti-HIV activity, can protect non-human primates from various types of HIV exposure, and is safe in humans without limiting toxicity.
On August 11, 2021, UCLA researchers published a clinical trial paper titled Cabotegravir for HIV Prevention in Cisgender Men and Transgender Women in the New England Journal of Medicine (NEJM).
This phase 2b/3 multi-center, randomized, double-blind, double-simulation, active controlled clinical trial compares the injection of Cabotegravir once every 2 months with daily oral TDF-FTC in men who have sex with men and trans women who have sex with men The safety and effectiveness of AIDS prevention in China.

The research team recruited 4566 volunteers (median age 26 years), of which 570 were transgender women (referring to their biological sex as male, but self-identified as female), and the rest were men who acted with men. These groups are high-risk groups of AIDS.
The researchers randomly assigned them 1:1 to receive two plans. Half of the people received an injection of Cabotegravir once every two months, and half of them received oral TDF-FTC every day. Then they were followed up for 153 weeks, and HIV testing and safety assessment were performed. The primary end point was an AIDS infection event.
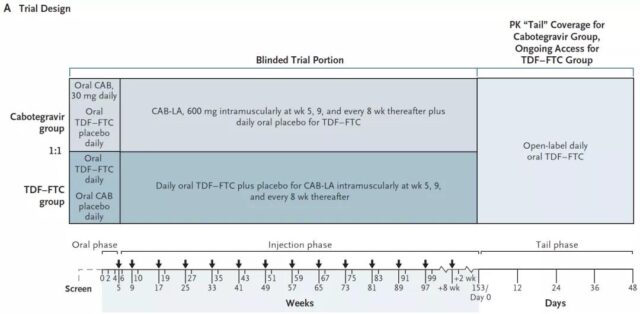
81.4% of participants in the Cabotegravir group and 31.3% of the participants in the TDF-FTC group reported injection site reactions, but no serious adverse events. A total of 52 participants were infected with AIDS, 13 in the Cabotegravir group (infection rate of 0.41/100 person-years), and 39 in the TDF-FTC group (infection rate of 1.22/100 person-years).
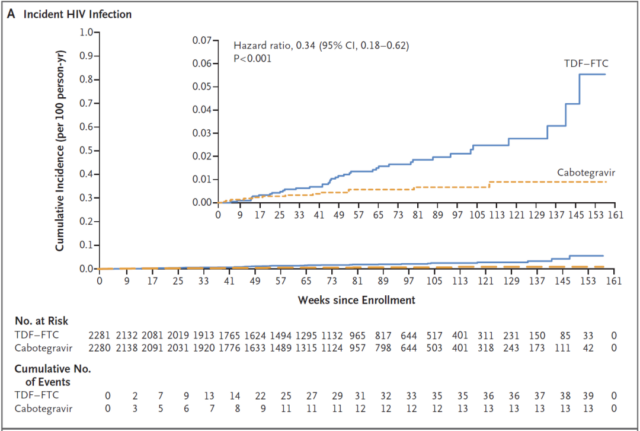
The results of this clinical trial show that long-acting Cabotegravir is better than daily oral TDF-FTC in preventing high-risk groups (men and trans women who have sex with men) from being infected with HIV.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



