FDA expert panel passes the half-dose booster of Moderna vaccine
- Why Lecanemab’s Adoption Faces an Uphill Battle in US?
- Yogurt and High LDL Cholesterol: Can You Still Enjoy It?
- WHO Releases Global Influenza Vaccine Market Study in 2024
- HIV Infections Linked to Unlicensed Spa’s Vampire Facial Treatments
- A Single US$2.15-Million Injection to Block 90% of Cancer Cell Formation
- WIV: Prevention of New Disease X and Investigation of the Origin of COVID-19
FDA expert panel passes the half-dose booster of Moderna vaccine
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
FDA expert panel passes the half-dose booster of Moderna vaccine.
Moderna half-dose booster
The FDA’s vaccine expert meeting discussed Moderna’s application for booster shot on October 14. Some previous studies have shown that Moderna is the COVID-19 vaccine with the highest level of effectiveness. It seems that even the strongest needs to be strengthened.
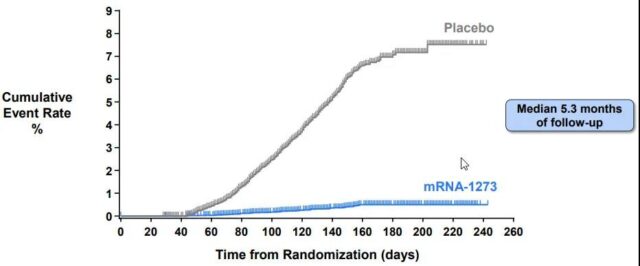
Moderna Phase III clinical trial tracked for more than 5 months without any decrease in vaccine effectiveness
Moderna is the same as Pfizer’s previous applicants. Both of them were people who hoped to get the first tow doses approved by the end of 2020-Pfizer is over 16 years old, Moderna is over 18 years old, and the third dose is given every 6 months.
However, Pfizer’s application was rejected and restricted to three groups: people over 65 years old, people under 65 years old but at high risk of severe illness, and people under 65 years old at high risk of infection due to occupational reasons. So Moderna also changed the target, limiting the applicants to these three.
This is the population approved by the FDA, and the specific practice population is subdivided by the CDC on this basis. For example, the CDC recommends Pfizer to classify people in nursing homes with elderly people over 65 years old when they are intensified.
Those who are under 65 years old are at higher risk of severe illness and subdivided them into 50 or less and 50 or less. Since the people applying for Moderna are the same as Pfizer, when the CDC discusses on October 21-22, it is estimated that the final group will be similar.
But the difference between Moderna and Pfizer is that Moderna’s application as an booster shot is a half-dose. The approved dose for the first two-dose is 100 micrograms, and the application for the third dose proposes 50 micrograms. This is not the same as Pfizer using the same dose. It also affected data collection.
From the data point of view, Moderna’s application for the booster shot is an immune bridging test, the test code is P201B. The experiment was divided into two groups. One group was vaccinated with 100 micrograms for the first two doses, 50 micrograms for the third dose, 171 people, and then the other group was 173 people, and the three doses were all 50 micrograms.
Miss a non-inferiority standard
Nowadays, the COVID-19 vaccine does not have a standard that says that the amount of antibodies will have a protective effect. Therefore, it cannot be said that the amount of antibodies that are enhanced will have a protective effect.
Therefore, the FDA has adopted non-inferiority standards for immune bridging. In other words, I know that your first tow doses are protective, which is proven in clinical trials.
The current booster shot of the same vaccine must prove that the immune response after the booster shot is at least not worse than the immune response induced by the first two shots in the Phase III clinical trial.
Switching to Moderna’s booster shot application, the booster shot immune response made by P201B should be compared with the first two doses in the Phase III clinical trial of Moderna, and it should not be worse than the latter.
How to prove that it is not bad? There are two comparison methods. One is neutralizing antibody titer. The standard is that the antibody titer one month after the third dose cannot be worse than the one month after the second shot. In the case of Moderna, in the P201B trial, just looking at the first tow doses of 100 micrograms group, 149 people have antibody data.
One month after the third dose, the average neutralizing antibody titer is 1802, while in the third phase clinical trial random More than 1,000 people were found, and the average neutralizing antibody titer was 1017. In this way, the antibody of the third stitch is 1.8 times that of the first two stitches, and the confidence interval is 1.5-2.1 times, which meets the non-inferiority standard.
It should be noted that immunological data can only use the first tow doses of 100 micrograms in the P201B trial, because the first tow doses of 100 micrograms in the phase III clinical trial and the actual approval. In addition, the three doses are all 50, which is different from the actual situation and cannot be used for comparison.
In addition to this indicator of increased antibody titer to be non-inferior, there is also an indicator of antibody positive reaction ratio that needs to be non-inferior. This indicator is a bit of the antibody conversion rate seen in the news. Some vaccines emphasize the conversion rate of 100% when they promote their effectiveness.
So how is the conversion rate calculated? The standard set by the FDA is that after one dose, a person’s neutralizing antibody titer increases more than four times the titer before the injection, and the person is considered to have turned positive.
The positive antibody reaction ratio of the booster shot should not be 10% lower than the ratio achieved by the first two doses to qualify as non-inferiority.
This indicator is quite demanding. Because the conversion rate of the first two shots is compared with the baseline when there is no vaccine, it is easy to exceed 4 times, and the proportion of antibody positive is very high.
However, when the booster shot is given, it is compared with the neutralizing antibody titer before the booster shot. In case there are still a lot of neutralizing antibodies at that time, it must be increased by four times to be positive. The requirement is very high.
On this indicator, Moderna’s BOOSTER SHOT did not achieve the non-inferiority of the first two doses. The conversion rate of the first tow doses was 98.4%.
This is based on the more than 1,000 people in the Phase III clinical trial. The first tow doses of 100 micrograms in the P201B group had a 4-fold increase in neutralizing antibodies compared to before the booster shot, which was 87.9%. The difference is more than 10%, and it is not non-inferior.
Limited amount of data and questions
The conversion rate has not been non-inferior. It does not mean that Moderna’s booster shots are not effective, but it has increased. If you look at those people who have not increased four times, the average value of neutralizing antibodies before the booster shot is higher.
This is in line with the principle of the booster shot. If the baseline level is high, the enhancement effect may be limited.
Regarding the antibody level before the booster shot and the enhancement effect, Moderna put together the people with P201B, including those with 50 micrograms for the three doses, for analysis.
There were 265 people with a 4-fold increase in antibody. The average neutralizing antibody was 108 before the booster shot, and the average antibody was 1964 after the injection.
The number of people who did not reach 4 times was 29, with an average of 492 neutralizing antibodies before the fight and 1354 after the fight.
This shows that people with a small increase in antibodies not only have a small increase, but also have a small total number of antibodies in the end.
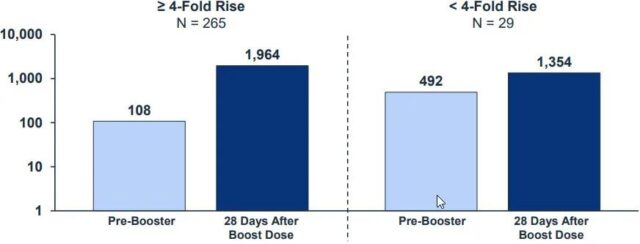
Attached picture: People who are unsuccessful in turning positive with enhanced acupuncture (right histogram) have high basal antibody (light blue) and low post-enhanced acupuncture antibody (dark blue)
Scientists at Moderna said that if the antibody is high, then when the booster shot is given, the protein expressed by the booster shot may be bound by the antibody, which will weaken the effect of the booster shot. This is more of an inference.
However, the observed phenomenon at least points to the timing of the booster shots. If you go to fight when the antibody is high, it not only means that the person himself may be well protected, it is not necessary, but the final vaccination effect may be limited.
Of course, this is a very small number and may not be representative. However, when Moderna does its own data presentation, it often puts all the subjects of P201B together, which is misleading. Because only half of the people who received 100 micrograms in the first two shots were involved.
Why do you do this? One possibility is to increase the number of people. After all, when Pfizer applied for 300 people, 171 was too ugly.
The other may also make some immunological data look better. Those with the first two doses of 50, it is very likely that the baseline before the third dose is low, and the antibody conversion rate is good.
In fact, the FDA only looked at the data of those 171 people, that is, the conversion rate did not reach the non-inferior part.
Moderna showed several two groups of more than 340 people together in this section, showing the conversion rate from different angles. Can. It was too complicated, and it stunned the expert group. At the questioning stage, it was pointed out that this was confusing, and then the FDA clarified that it only looked at the relevant data of 171 people.
In addition, 18 cases of infections occurred in these 171 people after the booster shot. Although only one case had symptoms, this raised a question mark on the possibility of the booster shot to resolve the infection.
The protection of asymptomatic infections was also questioned in the questioning session, because this is almost 10% of the infection rate.
If asymptomatic protection is good, it means that the infection rate can reach 30-40% without a vaccine, which is a bit impossible. Imagine.
In fact, it may be a combination of behavioral changes after vaccination and the high background infection rate. However, the main role of vaccines now is to prevent severe illness, so the lack of effectiveness in preventing infection does not affect the review of booster shot.
The experts can’t pass?
In general, Moderna’s booster shot data is very similar to Pfizer, and it also shows that the third doses increases antibodies. But Pfizer had the same problem with Moderna at the time, just how significant is this simple increase? Do you need it now? Pfizer did not give good answers to these questions, nor did Moderna.
Moderna still lacks even more. The first is that the number of people is too small, and the amount of data for 171 people is too small. This has been criticized by many experts, especially since there are basically no minorities in it, and it is completely unable to represent the general population of the United States.
Moreover, changing the dose to 50 micrograms has also been questioned which will cause confusion. Now it is necessary to distinguish whether a person vaccinated with Moderna is given a half dose or a full dose. In addition, not all the third dose of Moderna is half-dose.
The third dose of Moderna for immunosuppressed people is full-dose, which is more likely to cause confusion. Many scientific issues are unknown to this change. For example, is 50 micrograms good enough for immune memory?
But since Pfizer approved it before, this time Moderna finally passed the same crowd. Some experts bluntly said that because of Pfizer’s precedent, they had to agree with Moderna, but they were still very dissatisfied with the completeness of Moderna’s data.
Reference:
https://www.fda.gov/media/153089/download
https://www.fda.gov/media/153087/download
FDA expert panel passes the half-dose booster of Moderna vaccine
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



