First ADC drug for HER2-positive gastric cancer will redefine treatment!
- Why Botulinum Toxin Reigns as One of the Deadliest Poisons?
- FDA Approves Pfizer’s One-Time Gene Therapy for Hemophilia B: $3.5 Million per Dose
- Aspirin: Study Finds Greater Benefits for These Colorectal Cancer Patients
- Cancer Can Occur Without Genetic Mutations?
- Statins Lower Blood Lipids: How Long is a Course?
- Warning: Smartwatch Blood Sugar Measurement Deemed Dangerous
First ADC drug for HER2-positive gastric cancer will redefine treatment!
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
First ADC drug for HER2-positive gastric cancer will redefine treatment!
HER2-positive gastric cancer’s first ADC drug! Enhertu developed by AstraZeneca/Daiichi Sankyo enters review in EU: will redefine gastric cancer treatment!
Enhertu is the first ADC therapy approved for the treatment of HER2-positive gastric cancer, which significantly prolongs patient survival compared to nursing care.
Daiichi Sankyo recently announced that the European Medicines Agency (EMA) has accepted the HER2 targeting antibody conjugate (ADC) Enhertu (fam-trastuzumab) deruxtecan) category II change application: for the treatment of adult patients with locally advanced or metastatic HER2-positive gastric or gastroesophageal junction (GEJ) adenocarcinoma who have previously received an anti-HER2 regimen .
The EMA Committee on Medicinal Products for Human Use (CHMP) has initiated a scientific review of Enhertu’s application for the aforementioned new indications. Enhertu was developed by Daiichi Sankyo in cooperation with AstraZeneca .
The EU application, based on already published in the “New England Journal of Medicine” (NEJM) of DESTINY-Gastric01 2 clinical trials and DESTINY-Gastric02 2 During Last in 2021 the European Society for Medical Oncology (ESMO) Congress published clinical trials the result of.
The prognosis of gastric cancer is poor, especially in the advanced stages of the disease, when only 5%-10% of patients survive for 5 years. About one-fifth of gastric cancers are considered HER2 positive. The recommended first-line treatment for HER2-positive advanced or metastatic gastric cancer is a combination of chemotherapy and trastuzumab (HER2-targeted monoclonal antibody). Although the benefits of HER2 targeted therapy in the treatment of first-line metastatic gastric cancer have been fully confirmed, the disease will eventually progress, and second-line treatment options are limited, and new HER2 targeted therapies are urgently needed.
Enhertu is the first ADC drug approved to treat HER2-positive gastric cancer. The drug was approved in Japan and the United States in September 2020 and January 2021, respectively, for the treatment of patients with HER2-positive metastatic stomach or GEJ adenocarcinoma.
It is worth mentioning that Enhertu is the first to show that patients with HER2-positive metastatic gastric cancer who have previously received chemotherapy and anti-HER2 therapy have significantly prolonged overall survival compared with chemotherapy (Results of the DESTINY-Gastric02 trial, median OS: 12.5 months vs 8.4 months) HER2 targeted therapy.
Based on the convincing and strong efficacy in clinical trials , Enhertu will become the new standard of care for clinical treatment of such patients.
DESTINY-Gastric01 is an open-label, randomized phase 2 trial that enrolled 187 (including 149 cases in Japan) HER2-positive advanced gastric cancer or gastroesophageal junction adenocarcinoma (defined as: IHC3+ or IHC2+/ISH+) patients who had previously Received 2 or more regimens (including 5-FU, platinum-containing chemotherapy, trastuzumab) but the disease progressed.
In the study, patients were randomly assigned at a ratio of 2:1 and received Enhertu (6.4 mg/kg) or chemotherapy (paclitaxel or irinotecan monotherapy) selected by the research investigator, once every three weeks.
The results showed that the study reached the primary and key secondary endpoints: Compared with the chemotherapy group, the Enhertu treatment group achieved statistically significant and clinically significant improvements in objective response rate (ORR) and overall survival (OS).
The specific data are as follows: Among 175 evaluable patients (including 140 Japanese patients), the independent central review (ICR) assessment: The ORR of the Enhertu group was 51.3% (95%CI: 41.9-60.5%), and the chemotherapy group was 14.3 % (95%CI: 6.4-26.2%).
In a pre-specified interim analysis, the Enhertu group had a 41% lower risk of death compared with the chemotherapy group (HR=0.59; 95%CI: 0.39-0.88; p=0.0097). The median OS was 12.5 months in the Enhertu group and 8.4 months in the chemotherapy group.
DESTINY-Gastric02 is the first trial that specifically evaluates Enhertu in the treatment of Western gastric cancer patients. Data show that in patients with HER2-positive metastatic and/or unresectable gastric or gastroesophageal junction (GEJ) adenocarcinoma who have previously been treated with a trastuzumab regimen, Enhertu treatment provides clinically meaningful and durable The tumor is in remission.
The data released at the ESMO conference in 2021 showed that in the preliminary analysis, the overall response rate (ORR) of Enhertu (6.4 mg/kg) confirmed by the independent center review (ICR ) was 38% .
Among patients treated with Enhertu, 3 cases (3.8%) were observed in complete remission (CR) and 27 cases (34.2%) in partial remission (PR).
After a median follow-up of 5.7 months, the median duration of response (DoR) was 8.1 months (95%CI: 4.1-NE). The median progression-free survival (PFS) was 5.5 months (95%CI: 4.2-7.3). The confirmed disease control rate (DCR) was 81% (95% CI: 70.6-89.0).
These results are consistent with the results of the Phase 2 clinical trial of DESTINY-Gastric01 . In the DESTINY-Gastric02 trial, the overall safety of Enhertu was consistent with that in the DESTINY-Gastric01 trial.
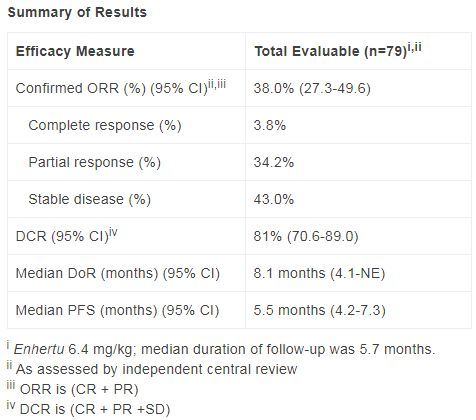
DESTINY-Gastric02 clinical trial data
Gastric cancer is the fifth most common cancer in the world and the fourth leading cause of cancer deaths. The 5-year survival rate for patients with advanced or metastatic disease is only 5%-10%.
In 2020, there will be approximately 1 million new cases of gastric cancer and 768,000 deaths worldwide.
The incidence of gastric cancer in East Asia is significantly higher, accounting for about half of all cases. Gastric cancer is usually diagnosed at an advanced stage, but even if the disease is diagnosed at an early stage, the survival rate is still very low.
About one-fifth of stomach cancers are HER2 positive. HER2 is a tyrosine kinase receptor growth-promoting protein that is expressed on the surface of many types of tumor cells, including breast cancer , gastric cancer, lung cancer and colorectal cancer. HER2 overexpression may be related to changes in a specific HER2 gene called HER2 amplification.
For HER2-positive advanced or metastatic gastric cancer, the recommended first-line treatment is chemotherapy + trastuzumab combination therapy.
Trastuzumab is an anti-HER2 drug, which has been proven to improve patient survival when combined with chemotherapy.
However, in patients with gastric cancer after receiving trastuzumab treatment, 29%-69% of patients have observed loss of HER2 expression, which suggests that reduced HER2 expression can lead to the progress of trastuzumab treatment and may be related to gastric cancer.
Related to the poor prognosis. For patients with metastatic gastric cancer, if progress occurs after the initial treatment with trastuzumab-based regimens, treatment options are limited, and there are no other HER2 targeted drugs available in many parts of the world.
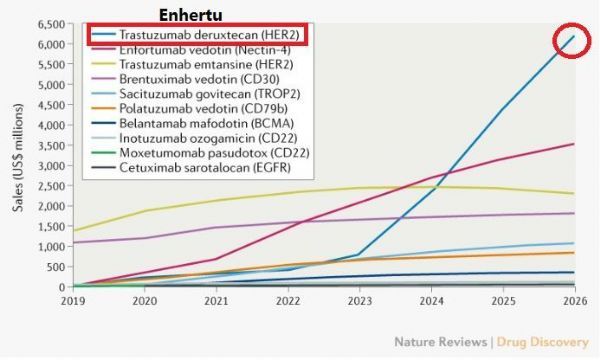
ADC drug sales forecast in 2026 (picture from document PMID 33762691)
Enhertu is a new generation of antibody-drug conjugate (ADC) that combines humanized HER2 monoclonal antibody trastuzumab (trastuzumab) with a new topoisomerase 1 through a 4-peptide linker.
The inhibitor exatecan derivatives (DX-8951 derivatives, DXd) are linked together to target the delivery of cytotoxic agents to cancer cells, and compared with usual chemotherapy, they can reduce the systemic exposure of cytotoxic agents.
In March 2019, AstraZeneca and Daiichi San total reached an immuno- oncology collaboration worth up to 6.9 billion U.S. dollars to jointly develop Enhertu for the treatment of cancer patients with various HER2 expression levels or HER2 mutations, including gastric cancer , Colorectal cancer, lung cancer, and breast cancer with low HER2 expression.
According to the agreement, the two parties will jointly develop and commercialize Enhertu on a global scale. Daiichi Sankyo will retain the exclusive rights to the Japanese market and will be solely responsible for manufacturing and supply.
Up to now, Enhertu has been approved for 2 indications:
(1) for the treatment of adult patients with HER2-positive metastatic breast cancer who have received two or more anti-HER2 drugs in metastatic disease;
(2) use For the treatment of adult patients with locally advanced or metastatic HER2-positive gastric or gastroesophageal junction (GEJ) adenocarcinoma.
The industry is very optimistic about Enhertu’s business prospects. In March of this year, the journal Nature Reviews Drug Discovery published an article (The oncology market for antibody-drug conjugates), pointing out that the global market for antibody-drug conjugates (ADC) is expected to be on the market by 2026 At over 16.4 billion U.S. dollars, Enhertu will rank first with sales of 6.2 billion U.S. dollars.
Reference:
Trastuzumab Deruxtecan Type II Variation Application Validated by EMA for the Treatment of HER2 Positive Advanced Gastric Cancer
First ADC drug for HER2-positive gastric cancer will redefine treatment!
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



