FDA approved Johnson & Johnson Darzalex Faspro for multiple myeloma!
- Why Lecanemab’s Adoption Faces an Uphill Battle in US?
- Yogurt and High LDL Cholesterol: Can You Still Enjoy It?
- WHO Releases Global Influenza Vaccine Market Study in 2024
- HIV Infections Linked to Unlicensed Spa’s Vampire Facial Treatments
- A Single US$2.15-Million Injection to Block 90% of Cancer Cell Formation
- WIV: Prevention of New Disease X and Investigation of the Origin of COVID-19
FDA approved Johnson & Johnson Darzalex Faspro for multiple myeloma!
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
FDA approved Johnson & Johnson Darzalex Faspro for multiple myeloma!
Takes only 3-5 minutes! FDA approved Johnson & Johnson Darzalex Faspro: combined with Kyprolis + dexamethasone for the treatment of multiple myeloma (MM)!
Darzalex Faspro is the first subcutaneously injected CD38-targeted antibody drug approved for the treatment of multiple myeloma.
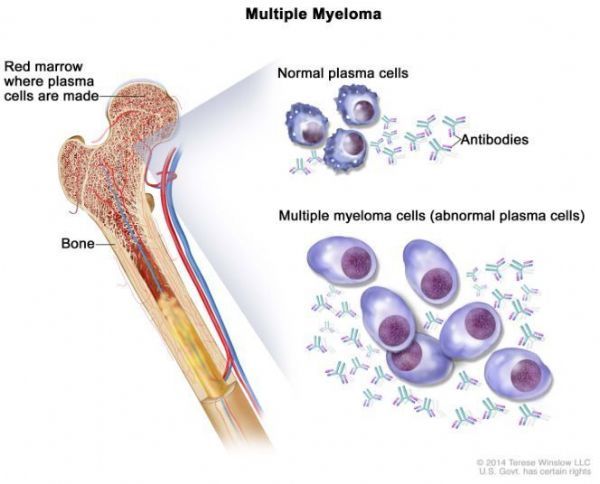
Multiple myeloma (picture from cancer.gov)
Johnson & Johnson (JNJ)’s Janssen Pharmaceuticals recently announced that the U.S. Food and Drug Administration ( FDA ) has approved the subcutaneous (SC) preparation of daratumumab – Darzalex Faspro (Generic Name: Daratumomab Injection [Subcutaneous Injection]) , combined with Kyprolis (carfilzomib, carfilzomib) and dexamethasone (Kd), used to treat past 1-3 lines Treatment of adult patients with relapsed or refractory multiple myeloma (R/R MM).
Darzalex Faspro is a subcutaneous injection (SC) preparation of Darzalex Faspro (Darzalex, Zhaoke®). Darzalex is the world’s first CD38-mediated, cytolytic antibody drug, and the first monoclonal antibody drug approved by the US FDA for the treatment of multiple myeloma (MM).
Its intravenous preparation (IV) was launched in 2015. It has become the backbone therapy for clinical treatment of MM and is widely used in first-line, second-line and multi-line treatment.
This approval also marks the 9th indication for Darzalex Faspro .
It is worth mentioning that Darzalex Faspro is currently the first and only subcutaneous injection of anti-CD38 monoclonal antibody drug approved for the treatment of multiple myeloma (MM) in combination with a series of standard treatment regimens.
Darzalex Faspro is administered in a fixed dose by subcutaneous injection, which only takes 3-5 minutes to complete.
Darzalex intravenous (IV) preparations are administered by intravenous drip, which usually takes several hours.
Compared with intravenous (IV) Darzalex+Kd regimen, the administration time of subcutaneous (SC) Darzalex Faspro+Kd regimen is greatly shortened, which will provide R/R MM patients with further options and treatment convenience, and will greatly Reduce the burden of disease management.
In August 2012, Genmab granted Janssen Biotech, Inc., a subsidiary of Johnson & Johnson, the exclusive global license to develop, produce and commercialize daratumumab (daratumumab).
Darzalex Faspro was developed using Halozyme’s ENHANZE® drug delivery technology, and the formula contains recombinant human hyaluronidase PH20 (rHuPH20).
This approval is based on the support of the ongoing Phase 2 non-random open label multicenter PLEIADES trial. The trial evaluated the clinical benefits of Darzalex Faspro in combination with 4 standard care treatments in patients with MM.
The updated data of the study has been announced at the 2020 American Society of Hematology (ASH) annual meeting.
The data show that: The remission rate of Darzalex Faspro+Kd regimen is similar to the remission rate of intravenous [IV] Darzalex+Kd regimen in the Phase 3 CANDOR study .
The results of the CANDOR study support the first regulatory approval for the combination of anti-CD38 monoclonal antibody and Kyprolis.
The PLEIADES study reached the primary endpoint, showing that the Darzalex Faspro+Kd regimen had an overall response rate (ORR) of 84.8% , and 77.3% of patients achieved a very good partial response (VGPR) or better response.
In this study, the safety of Darzalex Faspro+Kd regimen is consistent with the known safety of Darzalex Faspro, Kyprolis, and dexamethasone.
Multiple myeloma (MM) is an incurable hematological malignant tumors , characterized by repeated cycles of relapse and remission.
The disease is an extremely aggressive disease that affects plasma cells in the bone marrow, and these affected plasma cells replace normal cells in the bone marrow.
Although some patients with MM have no symptoms, most patients are diagnosed because of related symptoms.
These symptoms include fractures or pain, low red blood cell count, fatigue, high calcium levels, kidney problems, or infections.
Darzalex was first approved for marketing in November 2015.
The drug is the first CD38-mediated, cytolytic antibody drug approved in the world. It has broad-spectrum killing activity and can be targeted.
Combining with the transmembrane extracellular enzyme CD38 molecule highly expressed on the surface of multiple myeloma and a variety of solid tumor cells, it induces the rapid death of tumor cells through a variety of immune-mediated mechanisms, including complementary dependent cytotoxicity (CDC) , Antibody-dependent cell-mediated cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP) and through apoptosis (apoptosis).
In addition, Darzalex has also been shown to be able to target immunosuppressive cells in the tumor microenvironment to exhibit immunomodulatory activity.
Kyprolis was first approved for marketing in July 2012. The drug is an intravenously administered irreversible proteasome inhibitor.
The proteasome plays an important role in cell function and growth, degrading damaged or no longer needed proteins. Kyprolis has been shown to block the proteasome, causing excessive accumulation of proteins in the cell. In some cells, Kyprolis can cause cell death, especially multiple myeloma cells, because such cells are more likely to contain higher amounts of abnormal proteins.
Currently, Darzalex and Kyprolis have become important basic therapies for the treatment of multiple myeloma (MM) .
The combination of two powerful targeted drugs, Kypropris (proteasome inhibitor) and Darzalex (anti-CD38 monoclonal antibody), represents a new method for the treatment of relapsed or refractory multiple myeloma.
FDA approved Johnson & Johnson Darzalex Faspro for multiple myeloma!
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



