Small molecule drugs in tumor immunity
- Aspirin: Study Finds Greater Benefits for These Colorectal Cancer Patients
- Cancer Can Occur Without Genetic Mutations?
- Statins Lower Blood Lipids: How Long is a Course?
- Warning: Smartwatch Blood Sugar Measurement Deemed Dangerous
- Mifepristone: A Safe and Effective Abortion Option Amidst Controversy
- Asbestos Detected in Buildings Damaged in Ukraine: Analyzed by Japanese Company
Small molecule drugs in tumor immunity
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- What is the difference between Atorvastatin and Rosuvastatin?
- How long can the patient live after heart stent surgery?
Small molecule drugs in tumor immunity. Immunotherapy, which uses immune pathways to treat cancer, is rapidly becoming a recognized cancer treatment after surgery, chemotherapy and radiotherapy.
Immunotherapy strategies include antibody-based “checkpoint” suppression, adoptive T cell therapy, and therapeutic vaccination. Use monoclonal antibodies (mAb) against cytotoxic T lymphocyte-associated protein 4 (CTLA-4), programmed death receptor 1 (PD-1) or programmed death ligand 1 (PD-L1) for checkpoint blocking In the end, significant breakthroughs have been made in cancer immunotherapy.
Despite its advantages, immunotherapy has only been successful in a small number of patients, and the biomarkers that broadly predict its efficacy have yet to be determined. The immune response to cancer is usually limited by three main bottlenecks: (i) the recognition of tumor cells as “non-self”, (ii) peripheral immune tolerance, and (iii) immunosuppression in the tumor microenvironment (TME). Immunotherapy itself, or in combination with other strategies, is best to overcome these bottlenecks. So far, various combination therapies have been tested, but the effects are limited due to lack of synergy or unacceptable toxicity.
Here, the use of small molecule therapies may prove to be helpful because these drugs have many advantages over monoclonal antibodies. In particular, small molecules with shorter half-lives are conducive to acute and reversible effects, and may reduce long-lasting systemic side effects. Unlike antibodies, small molecules usually target intracellular proteins and have different toxicity characteristics, making them suitable candidates for combination therapy.
In addition, they can be produced at a lower cost than antibodies, and they can usually be administered orally. Therefore, new strategies based on molecular insights from immunological and oncology processes are needed to increase the potential of small molecules in immunotherapy. The focus is on small molecules that are expected to increase the success rate of checkpoint blockade against cancer.
1 Small molecule drugs targeting PD-1 or PD-L1
The PD-1/PD-L1 functional axis inhibits TCR and CD28 signaling, thereby limiting the optimal priming of tumor-specific T cells and their anti-tumor activity. Currently, this functional axis is mainly targeted by antibodies; however, small molecule PD-1/PD-L1 antagonists may help reduce toxicity.
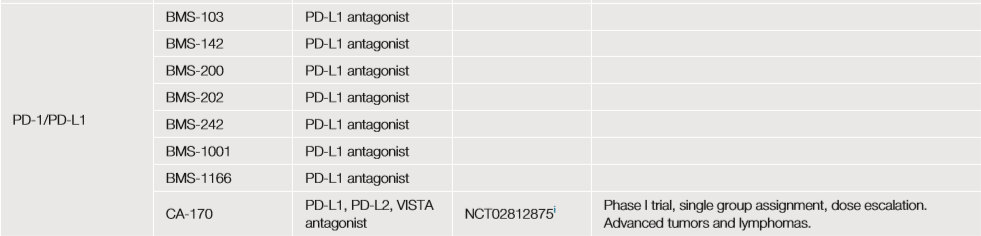
Some of them seem to work through a new dimer locking mechanism (such as BMS-103, -142, -200, -202, -242, -1001, and -1166), which have good effects in vitro. Another small molecule antagonist (CA-170) for PD-L1, PD-L2 and VISTA is currently in a phase 1 dose-climbing trial for patients with advanced solid tumors and lymphoma (300 subjects) (NCT02812875 ).
However, the development of small molecules targeting the PD-1/PD-L1 pathway lags behind monoclonal antibodies. This is due to the hydrophobicity of the PD-1/PD-L1 interface. It is very challenging to design high-affinity small molecule blockers. .
2 Therapeutic vaccines
Therapeutic vaccines are designed to induce tumor-specific CD8+ T cells to produce CTL responses. In order to obtain the best CTL activation, the help of CD4+ T cells is needed. Therefore, therapeutic vaccines include specific antigens for CD4+ and CD8+ T cells, as well as compounds that activate DC cells.
The main strategy is to use synthetic long peptides (SLP) (about 20-40 amino acids in length) or antigen-encoded mRNA or DNA, including MHCI and MHCII epitopes, to ensure CD8+CTL initiation and CD4+T cell assistance , So as to obtain a strong CTL response. These vaccines have shown certain therapeutic prospects in the treatment of early-stage viral cancers.
In addition, the recent ongoing phase Ib randomized glioblastoma trial (NCT02287428) shows that according to single-cell TCR analysis, inoculation with multi-epitope and personalized neoantigens can successfully induce neoantigen-specific CD4+ and CD8+ T cells in the tumor Immune response.
However, all patients participating in the study eventually relapsed, indicating that tumor-related immunosuppression and other challenges are an important and ongoing bottleneck.
3 Small molecule drugs targeting Toll-like receptors
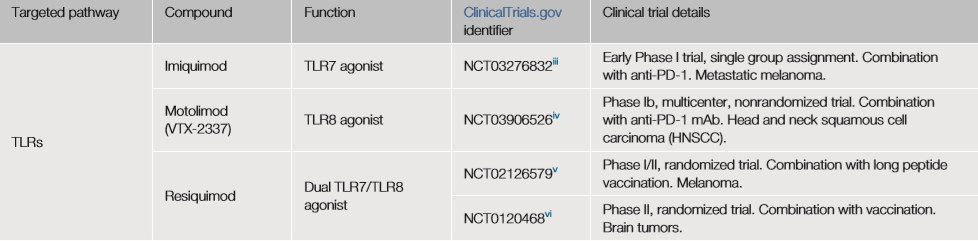
The first small molecule immuno-oncology drug approved by the FDA for the treatment of basal cell carcinoma is Imiquimod, an imidazoline derivative commonly used to treat genital warts.
Imiquimod targets toll-like receptor 7 (TLR7), a pattern recognition receptor (PRR) that binds to conserved PAMPs, such as double-stranded RNA, lipopolysaccharide, or unmethylated CpG-DNA.
Most TLRs are expressed on the cell surface, but TLR3, 7, 8 and 9 are mainly located in endosomes. The small molecule TLR8 agonist motolimod (VTX-2337) exhibits anti-tumor activity in recurrent or metastatic head and neck squamous cell carcinoma (HNSCC) by stimulating natural killer cells (NK) and enhancing antibody-dependent cell-mediated toxicity.
Motolimod combined with cetuximab (an anti-EGFR antibody) or conventional chemotherapy resulted in a decrease in the number of Tregs in TME and an increase in the number of circulating EGFR-specific CD8+ T cells. Compared with cetuximab or chemotherapy, progression-free survival Rate (PFS) and overall survival increased.
Imiquimod, motolimod and resiquimod (targeting TLR7 and TLR8) are currently undergoing a series of clinical trials (NCT03276832), (NCT0397626), (NCT02126579), (NCT01204684) for the treatment of solid tumors, usually as adjuvants for vaccination.
Therefore, research on small molecules for other TLRs continues, and high-throughput screening of drug libraries is usually used in cell-based analysis.
Other PRRs, such as NOD-like receptors (NLRs), C-type lectin receptors (CLRs), or RIG-I-like receptors (RLRs) have been less studied, but agonists targeting these families may enhance immune reactivity. in development.
4 Small molecule drugs targeting cGAS/STING pathway
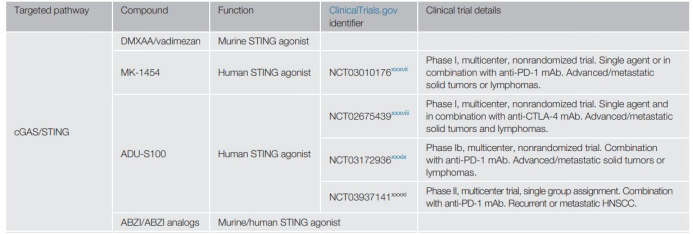
STING is a PRR on the endoplasmic reticulum membrane, which binds to cyclic dinucleotides in the cytoplasmic DNA transformed by cGAS. The activation of the cGAS/STING pathway leads to the production of type I IFN, which promotes the activation of DC and the initiation of T cells.
The results of animal experiments in tumor-bearing mice suggest that STING may be an important target for tumor immunotherapy. The STING signaling pathway is regulated by exonucleotide pyrophosphatase/phosphodiesterase-1 (ENPP1), which hydrolyzes cGAMP, thereby controlling the activation of the signaling cascade.
Therefore, people try to activate the STING pathway by inhibiting ENPP1. However, there are other research reports that cGAS/STING signal can induce indoleamine-2,3-dioxygenase (IDO1; a tryptophan catabolic enzyme that can induce immune suppression and immune evasion) and inhibit homologous Mediated DNA repair, thereby inhibiting the immune response of Lewis lung cancer mouse model and promoting tumor growth.
These studies show that before we fully understand the effects of STING agonist therapy, more studies are needed to prove the role of cGAS/STING pathway in tumor immunity.
5 Immunogenicity of standard care treatment
Radiotherapy and chemotherapy can directly kill tumor cells, but can also enhance anti-tumor immunity. The prevailing view is that these treatments may cause immunogenic cell death, which is characterized by the release of tumor antigens and danger signals (such as cytoplasmic DNA), which can activate DCs through PRR, such as TLRs and cGAS/STING. When the standard of care treatment follows immunotherapy, significant effects have been reported in preclinical animal models.
At present, according to the results of the Phase III KEYNOTE-189 clinical trial (NCT02578680), the FDA has approved the use of pembrolizumab (anti-PD-1 monoclonal antibody) in combination with cisplatin and pemetrexed as a treatment for metastatic non-small cell lung cancer (NSCLC). First-line treatment.
In addition, anthracyclines (such as doxorubicin) can induce the production of type I interferon in mouse models of fibrosarcoma, and selectively deplete immunosuppressive myeloid-derived suppressor cells in mouse breast cancer models ( MDSC), thereby inhibiting the progression of tumors in the body. The recent phase II single-center tension trial (NCT02499367) showed that compared with anti-PD-1 alone, cisplatin (OR 23%) or doxorubicin (OR 35%) combined with nivolumab (anti-PD-1 monoclonal antibody) Use can improve the treatment effect of patients with triple negative breast cancer (TNBC).
Similarly, atezolizumab (anti-PD-L1 monoclonal antibody) combined with paclitaxel (a chemotherapy drug that blocks mitosis by stabilizing microtubules) has now been approved by the FDA for the treatment of locally advanced or metastatic TNBC. This is based on the results of the Phase III IMpassion130 trial (NCT02425891), which shows that the PFS of combination therapy is better than chemotherapy alone.
Other chemotherapy drugs that enhance the effect of checkpoint inhibitors currently in clinical trials include: cyclophosphamide; platinum drugs, such as oxaliplatin; and PARP inhibitors olaparib (NCT02484404; NCT02634), talazoparib (NCT03964532; NCT03330405), rucaparib (NCT03639935) and veliparib.
In addition, various cyclin-dependent kinase 4 and 6 inhibitors (CDK4/6i), including palbociclib and abemaciclib, and anti-androgen drugs enzalutamide, bicalutamide (NCT03650894), GTX-024 (NCT02971761) And fulvestrant (NCT03280563).
6 Small molecule drugs targeting IDO1
Small molecule drugs can also be used to specifically target inhibitors to induce or restore the immune reactivity of TME. One such TME target includes IDO1.
The immunosuppressive effects of IDO1/kynurenine include the expansion of Treg cells and the recruitment of MDSCs. IDO1 deprives the tryptophan required for CTL activation and antagonizes CD8+ T cell effector function through PD-1 expression.
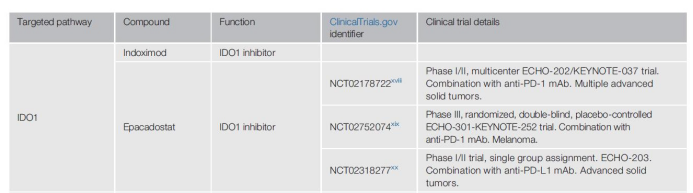
Indoximod is the first IDO1 inhibitor tested in humans, but the results are confusing because this compound may actually inhibit mTORC1, the downstream effector of IDO1.
Epacadostat is a more specific IDO1 inhibitor, used in combination with pembrolizumab (anti-PD-1 monoclonal antibody), in the I/II phase ECHO-202/KEYNOTE-037 trial (NCT02178722) for multiple advanced solid tumors The patient showed good results, but the anti-PD-1 efficacy was not increased in the phase III clinical trial (NCT02752074) in patients with melanoma.
Similarly, in the phase I/II ECHO-203 trial (NCT02318277), Epacadostat and anti-PD-L1 monoclonal antibodies were well tolerated, but there was no combined effect in these patients. Therefore, many pharmaceutical companies have stopped or are reducing the development of IDO1 inhibitors, which greatly reduces the early clinical development of such small molecules.
7 Small molecules targeting the prostaglandin pathway
One of the driving factors of IDO1 expression is cyclooxygenase 2 (COX-2), which is an understudied target in tumor immunotherapy, but it is also a common target of non-steroidal anti-inflammatory drugs (NSAIDs).
COX-2 catalyzes the synthesis of prostaglandin, a lipid compound involved in the response to injury and inflammation. This enzyme is expressed in several cancers, so celecoxib (a non-steroidal anti-inflammatory drug that can inhibit COX-2 and IDO1) is being used for cancer treatment.
A study developed an analog of celecoxib, which has a strong cytostatic effect on melanoma and colon cancer cell lines in vitro. The simultaneous inhibition of COX-2 and EGFR has a synergistic effect on cell proliferation and apoptosis of NSCLC cell lines in vitro.
In the Lewis lung cancer mouse model, the dual inhibitory effect of melafolone (a naturally occurring flavonoid) on COX-2 and EGFR can improve PD-1 blockade by normalizing blood vessels and down-regulating PD-L1. These studies demonstrate the potential of combination therapy to target multiple tumor-related molecules at the same time.
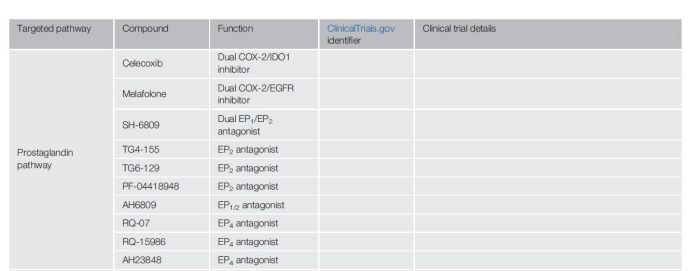
Downstream of the COX-2 signaling pathway are G protein-coupled receptors EP2 and EP4, which bind to prostaglandin E2 (PGE2). Prostaglandins send signals through the EP2 and/or EP4 pathways to block DC activity and redirect DC differentiation to inhibit phenotype and inhibit macrophages, which is of great significance in establishing an immunosuppressive environment.
Therefore, people’s interest in small molecule antagonists for these receptors is increasing, and various EP2 and EP4 antagonists are under development (for example, AH6809, AH23848, TG6-129, TG4-155, PF-04418948, RQ-07 and RQ-15986).
The combined application of dual inhibition of EP2 and EP4 and checkpoint inhibitors showed that in vitro epithelial ovarian cancer, tumor-derived CTL increased the production of antigen-specific pro-inflammatory cytokines.
Therefore, controlling prostaglandin signaling in TME may improve anti-tumor immunity, although extensive testing is clearly necessary.
8 Small molecule drugs targeting arginine metabolism
Arginase is another potential therapeutic target for TME. This ubiquitous manganese-containing enzyme catalyzes the hydrolysis of L-arginine to L-ornithine and urea, and plays an important role in all aspects of inflammation. There are two subtypes of arginase in mammals: cytoplasmic arginase Ⅰ (ARG Ⅰ) in the liver and arginase Ⅱ (ARG Ⅱ) in the mitochondrial matrix.
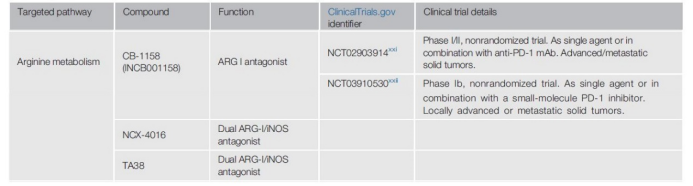
In TME, MDSCs and TAMs can release a large amount of ARG I to the extracellular space to locally consume arginine concentration, thereby impairing the transmission and proliferation of TCR signals. In T cell co-culture, the ARG I inhibitor CB-1158 (INCB001158) blocked the bone marrow cell-mediated immune suppression of T cell proliferation and reduced tumor growth in different mouse models.
In addition, Compared with the control group, in the CB-1158 treatment group, IFN-induced gene expression increased, inflammatory cytokine expression increased, and tumor-infiltrating NK cells and CD8+ T cells increased. Currently, two early clinical trials (NCT02903914; NCT03910530) for the drug as a single drug and in combination with anti-PD-1 monoclonal antibodies and small molecule inhibitors for advanced/metastatic solid tumors are ongoing.
Another enzyme that is abundantly expressed in MDSCs and TAMs is nitric oxide synthase (iNOS). iNOS hydrolyzes L-arginine to nitric oxide (NO), which then inhibits T cell function by interfering with the JAK3-STAT5 signaling pathway. When ARG-I is inhibited, iNOS has more substrates to produce NO, which leads to immunosuppression. To overcome this, dual ARG I/iNOS inhibitors, such as NCX4016 and TA38, have recently been developed and will be tested in the near future.
9 Targeting Tregs in TME
Tregs can express extracellular nucleotidase CD39 and CD73; membrane molecules that produce adenosine by dephosphorylation of ATP.
Adenosine can then bind to A2A or A2B receptors on the surface of conventional T cells and was found to inhibit CD8+ T cell infiltration in mouse models of melanoma. Adenosine can also bind to the A2A receptor on Tregs, leading to the expansion of the Treg population and enhancing its immunosuppressive effect in vitro.
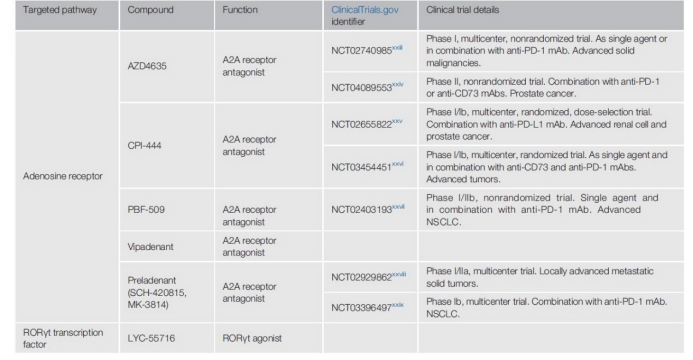
In order to alleviate Treg-mediated TME inhibition, small molecule A2A antagonists have been developed, such as CPI-444, AZD4635, vipadenant, preladenant (SCH-420815, MK3814, MSD) and PBF-509.
These compounds are currently undergoing phase I and phase II clinical trials, alone or in combination with anti-PD-1 or anti-PD-L1 inhibitors for various solid tumors (NCT02740985, NCT0408953, NCT02655822, NCT03454451 and NCT02403193).
Another Treg target is the retinoic acid receptor-related orphan receptor γ (RORγt), a transcription factor involved in the IL-17 pathway of T cell pro-inflammatory. RORγt agonists can induce the production of cytokines and chemokines, reduce the proliferation of Tregs, and reverse the immunosuppressive effect of tumor cells.
Synthetic small molecule RORγt agonists promote the activity, proliferation and survival of Th17 (CD4+) and Tc17 (CD8+) cells, and enhance Th17 effector function in mouse models of adoptive T cell therapy.
Two phase II clinical trials are testing the effects of these agonists, one as a single drug to assess safety and tolerability (NCT02929862), and the other to assess the safety/tolerability of NSCLC alone or in combination with anti-PD-1 monoclonal antibodies Sex (NCT03396497).
The results of these tests are difficult to predict because Th17 cells are associated with poor prognosis in many cancer types. In these cases, RORγt antagonists may provide therapeutic benefits.
However, because RORγt has a large lipophilic ligand binding domain, the design of inhibitors is very complicated.
In addition, stimulation of RORγt can promote autoimmune diseases, such as inflammatory bowel disease. Therefore, considering that the “classical” checkpoint inhibitors anti-CTLA-4 and anti-PD-1 antibodies can also induce autoimmunity, it is necessary to remind that this type of combination may cause strong side effects.
10 Small molecules targeting chemokine receptors
The function of chemokines and their receptors guide tumor cells to migrate to the metastasis site and direct immune cells to specific tissues.
The chemokine receptor CXCR4 is often activated in cancer cells and participates in epithelial-mesenchymal cell transformation, invasion, metastasis, and tumor vascularization.
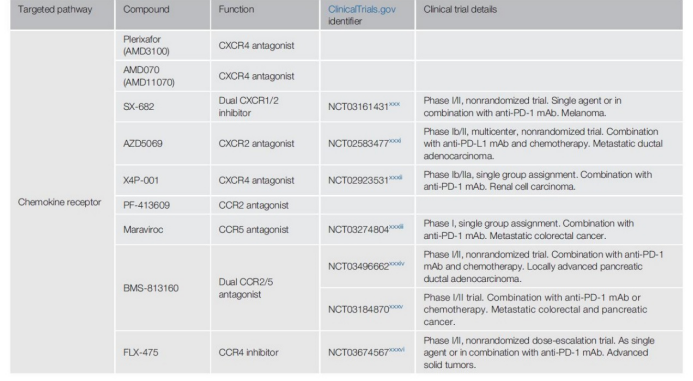
A series of small molecule antagonists of chemokine receptors have been developed. One of the most famous, plerixafor (AMD3100) has been reported on the efficacy of acute lymphoblastic leukemia and relapsed acute myeloid leukemia.
In a xenograft mouse model of small cell lung cancer, plerixafor combined with chemotherapy can reduce the growth of primary tumors and inhibit metastasis.
The inhibitory effect of plerixafor on CXCR4 counteracts the upregulation of CXCL12-dependent PD-L1 in TME and the recruitment of immunosuppressed Tregs and M2 macrophages.
This study used a mouse model of hepatocellular carcinoma to show that, compared with the control group, CXCR4 inhibition combined with anti-PD-1 mAb and sorafenib inhibited tumor growth, reduced lung metastasis, and improved animal survival.
The chemokine receptor CXCR2 is overexpressed in various cancers and is associated with the poor prognosis of human pancreatic ductal adenocarcinoma patients.
In mouse models of pancreatic cancer, breast cancer, and colorectal cancer, CXCR2 inhibition can prevent bone marrow mesenchymal stem cells from entering TME, so some people suggest that it be used to increase tumor sensitivity to immunotherapy.
Currently, a phase I/II non-randomized trial is recruiting melanoma patients to test the safety and effectiveness of the dual CXCR1/2 inhibitor SX-682 as a single drug or in combination with an anti-PD-1 monoclonal antibody (NCT03161431 ).
Other chemokine receptors that target small molecules are being evaluated as single drugs or combined checkpoint blockers in preclinical and clinical trials.
These include CXCR2 antagonist AZD5069 (NCT02583477), CXCR4 inhibitor X4P-001 (NCT02923531), CCR2 inhibitor PF-413609, CCR5 inhibitor maraviroc (NCT03274804), CCR2/5 antagonist BMS-813160 (NCT03496662), (NCT03184870) And CCR4 inhibitor FLX475 (NCT03674567).
Outlook
The field of tumor immunotherapy is explosively developing, and a new stage of small molecules’ directed and specific regulation of immune response is taking shape. There are many exciting developments, and it is likely that in the near future, new small molecules will be used in combination with anti-PD-1/PD-L1 or anti-CTLA4 blocking monoclonal antibodies.
The new therapeutic strategy of inducing specific protein degradation through protein degradation targeted chimera (PROTACs) technology will significantly increase the number of potential small molecule targets, and will greatly expand the options for manipulating immune responses.
These new technologies, along with other new drug developments, are expected to further expand the library of cancer treatment methods in the future.
Targeting PD-1/PD-L1 and CTLA-4 signaling pathways in immunotherapy can induce effective anti-tumor CTL responses in a variety of cancer patients. However, only some patients respond, and the available treatment combinations are usually consistent with serious adverse events.
Therefore, while reducing toxicity, new treatment options are essential to further improve the efficacy of tumor immunotherapy. And small molecule drugs provide an opportunity to improve the success rate of treatment. Compared with most antibodies, small molecules can easily penetrate into tissues, so they can directly act on extracellular and intracellular targets to improve anti-tumor immunity.
In addition, their half-life is usually very short, which reduces their chances of adverse reactions. Because of these characteristics, the development of small molecule-based strategies in the field of cancer immunotherapy has aroused widespread interest.
How to choose a combination of chemotherapy and immunotherapy based on known molecular mechanisms is a persistent challenge in this regard.
In addition, the focus should be on optimizing the dosage and timing of these combination therapies to maximize the synergy.
Chemoimmunotherapy has many targets to be evaluated, and only a few combinations have been evaluated or are currently being tested. Therefore, the future of chemoimmunotherapy is still broad and exciting.
(source:internet, reference only)
Disclaimer of medicaltrend.org



