Tumor lipid metabolism and oral checkpoint drugs
- Aspirin: Study Finds Greater Benefits for These Colorectal Cancer Patients
- Cancer Can Occur Without Genetic Mutations?
- Statins Lower Blood Lipids: How Long is a Course?
- Warning: Smartwatch Blood Sugar Measurement Deemed Dangerous
- Mifepristone: A Safe and Effective Abortion Option Amidst Controversy
- Asbestos Detected in Buildings Damaged in Ukraine: Analyzed by Japanese Company
Tumor lipid metabolism and oral checkpoint drugs
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- What is the difference between Atorvastatin and Rosuvastatin?
- How long can the patient live after heart stent surgery?
Tumor lipid metabolism and oral checkpoint drugs.
With the in-depth research on tumor lipid metabolism inhibition and the development of oral PD-L1 small molecule inhibitors, Gerry Pharmaceuticals (1672.HK) recently announced its investment in tumor lipid metabolism and oral checkpoint inhibitor research and development. upgrade.
It is understood that the US Phase II clinical trial of ASC40 (TVB2640), a fatty acid synthase (FASN) inhibitor under Gerry’s, has confirmed that its combination with anti-angiogenic drugs has shown good results in the treatment of patients with high-grade astrocytoma recurrence for the first time The efficacy and safety.
In the future, FASN inhibitors are expected to become a new multi-tumor treatment platform, and provide more efficient and safe treatment modes through combined application with oral PD-L1 small molecule inhibitors.
FASN inhibitor: A new option for tumor lipid metabolism pathway therapy
In recent years, tumor metabolism pathway has become one of the research directions that have attracted much attention in the field of new anti-tumor drug research and development.
Compared with normal cells, tumor cells usually undergo energy metabolism reorganization to meet their needs for rapid proliferation.
For example, tumor cells can induce the overexpression and activation of related lipases, promote the de novo synthesis of fatty acids, and obtain more phospholipids for construction. Cell membrane [1,2].
Fatty acid synthase (FASN) is a key enzyme in the process of fatty acid synthesis. Since the 1990s, studies have successively found that FASN is present in solid tumor tissues such as breast cancer, prostate cancer, colon cancer, lung cancer, bladder cancer, ovarian cancer, gastric cancer, endometrial cancer, kidney cancer, skin cancer, and esophageal cancer. It is expressed at a high level and is associated with poor prognosis or treatment resistance [3,4].
Further mechanism studies have found that when exogenous fatty acids cannot meet the supply, tumor cells can increase the utilization of endogenous fatty acids, and FASN plays an important role in the synthesis of endogenous fatty acids [5,6]. It can be seen that FASN is the key to obtaining “grain and grass supply” in the process of tumor proliferation and metastasis.
Inhibition of FASN can prevent the signal transduction network required for tumor growth and proliferation by destroying the cell membrane structure, including RTK, PI3K-Akt-mTOR and MAPK signaling pathways [7].
ASC40 (TVB2640) is a world’s first (First-In-Class), oral FASN inhibitor, and its efficacy and safety in the phase II trial for the treatment of non-alcoholic steatohepatitis (NASH) have been affirmed.
In view of the key role of FASN in tumor energy metabolism, studies have applied ASC40 (TVB2640) to the treatment of solid tumors and showed good anti-tumor activity [8].
At the European Medical Oncology Annual Conference in 2020, a phase II trial initiated by investigators showed that the objective response rate (ORR) of ASC40 (TVB2640) combined with bevacizumab in the treatment of patients with first recurrence of high-grade astrocytoma reached 65 % (Complete remission rate + partial remission rate: 20%+45%), the 6-month progression-free survival rate was 47%, which was significantly better than the historical control (BELOB 16%, P=0.01), and it was well tolerated, Most of the adverse reactions were grade 1 or 2, and there were no treatment-related adverse reactions of grade 4-5 [9].
Small molecules, big prospects: Oral PD-L1 small molecule inhibitors are coming
Since entering the 21st century, programmed death receptor 1 (PD-1) and its ligand (PD-L1) can be described as hot targets in the field of tumor immunotherapy.
At present, many PD-1/PD-L1 antibody drugs have entered clinical practice and achieved great success. The indications cover almost all types of solid tumors.
A series of innovative pharmaceutical companies including Junshi Bio, Cinda Bio, BeiGene, and Hengrui Pharmaceuticals have also become popular star companies in the capital market by virtue of the breakthrough in the commercialization of PD-1 antibody therapy, and have achieved corporate output value. And the “double harvest” of market value.
However, antibody PD-1/PD-L1 inhibitors also have certain limitations, such as:
① The limited tissue penetration of macromolecules leads to low ORR of monotherapy and relatively low permeability of the blood-brain barrier. Poor;
②The antibody has a long half-life and strong immunogenicity, which can cause a variety of clinical side effects, especially the strong immune response caused by the excessive activation of the immune system. The long half-life limits the flexibility of combined use with other drugs;
③The administration method based on intravenous injection is far less compliance than oral anti-tumor therapy. In order to further solve the above problems, the development of oral bioavailable small molecule PD-1/PD-L1 inhibitors with better tissue penetration may open a new era of immunotherapy.
At present, the mechanism of PD-L1 small molecule inhibitors is mainly through small molecule compounds that bind to the PD-L1 ligand on the tumor surface to form dimers and are endocytosed by cells, thereby blocking PD-1 on the surface of T cells from the tumor.
The PD-L1 interaction on the surface relieves the immunosuppression of T cells [10].
Animal experiments have preliminarily proved the efficacy and safety of small molecule PD-L1 inhibitors, and individual small molecule PD-L1 inhibitors have started clinical trials [11].
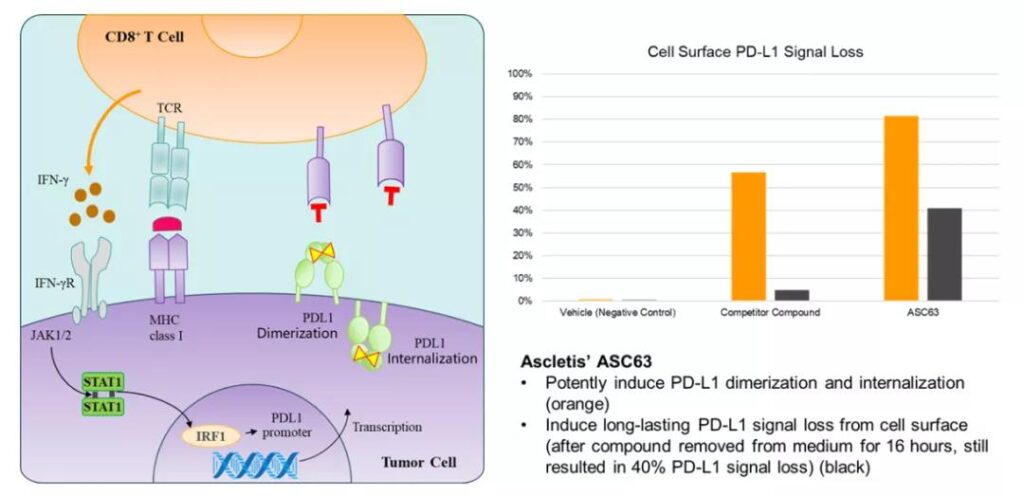
The anti-tumor mechanism of small molecule PD-L1 inhibitors (left) and the loss rate of PD-L1 on the cell surface produced by ASC63 [12]
In the field of viral hepatitis, the world’s first phase II trial of subcutaneous injection of PD-L1 antibody ASC22 in the treatment of chronic hepatitis B by Gallée has shown good efficacy and safety, and has entered a later clinical trial. Based on a deep understanding of the development of PD-L1 antibody drugs, Gerry has further planned the development of oral PD-L1 small molecule inhibitors (ASC61 and ASC63).
Pre-clinical data shows that the small molecule compounds developed by Gallite not only induce a stronger PD-L1 endocytosis effect than competing products, but also have a stronger endocytosis effect than competing products. (Figure 2) [12].
FASN inhibitor + oral PD-L1 small molecule inhibitor: Commercial considerations of the Golly Oncology Pipeline
As a biotechnology company with innovative R&D as its core driving force since its inception, Gerry is committed to the research and development of new anti-tumor drugs to further meet the clinical needs of cancer patients.
With the gradual clarification of the anti-tumor mechanism of FASN in lipid metabolism in recent years, and the maturity of PD-L1 small molecule inhibitor research and development technology, Gerry began to concentrate on the development of new tumor drugs and promote new FASN inhibitors (ASC40, ASC60). ), the development of oral PD-L1 small molecule inhibitors (ASC61, ASC63), and the mechanism and clinical research of FASN inhibitors combined with PD-L1 for the treatment of more types of tumors will be carried out.
The abnormal metabolism of tumors is not only manifested in the tumor cells themselves, but also in immune cells closely related to the effect of tumor immunotherapy.
Different immune cells have different requirements for metabolism, providing opportunities to regulate immune cell functions.
Selective intervention in metabolism, reducing the immunosuppression of the tumor microenvironment, and increasing the activity of effector cells can improve the effect of immunotherapy [13].
Studies have shown the influence of FASN on the tumor microenvironment.
For example, the de novo synthesis of fatty acids mediated by FASN contributes to the functional maturation of regulatory T cells (Tregs), and FASN loss in Tregs can inhibit tumor growth [14]; inhibit FASN It can partially restore the immune activity of tumor-infiltrating dendritic cells (TIDCs), thereby increasing the anti-tumor immune response [15].
Studies have also shown that FASN and PD-L1 levels in cisplatin-resistant non-small cell lung cancer (NSCLC) are significantly increased, and inhibiting or knocking out FASN can increase the cytotoxicity of cisplatin-resistant cells to natural killer (NK) cells.
Sensitivity, the mechanism may be that the downstream signaling molecule TGF-β1 of FASN is responsible for regulating the level of PD-L1 in cisplatin-resistant cells [16].
Therefore, inhibiting the FASN-TGFβ1-PD-L1 signal axis may improve the immunotherapy effect of cisplatin-resistant lung cancer.
In summary, FASN inhibitors not only intervene in the lipid metabolism pathway of tumors, but also have a basis for joint use with the current mainstream anti-angiogenesis (Angiogenesis), tumor-targeted therapy, and immunotherapy.
Tumor immunotherapy represented by the PD-1/PD-L1 antibody is an important treatment for tumors at the moment. It was once ranked as the top ten scientific breakthrough of the year by “Science”.
According to the analysis of the Frost & Sullivan report, the future will follow With the approval of more PD-1/PD-L1 monoclonal antibodies, the expansion of indications, and the increase in penetration, the global tumor immunotherapy market is expected to grow further, reaching a peak of US$79.8 billion in 2027. However, under the huge market prospect, the PD-1/PD-L1 innovative drug market is also facing considerable challenges.
First of all, the problem of high concentration of targets and indications has become increasingly apparent, and the homogeneity competition has become increasingly fierce.
In addition, although immunotherapy can be used as a broad-spectrum anti-tumor drug for a variety of tumor treatments, it is an indisputable reality that different tumors have very different responses to antibody drugs.
In tumor immunotherapy combined with lipid metabolic pathway therapy Anti-tumor immune response is expected to become a new strategy for tumor immunotherapy in the future.
The oral PD-L1 small-molecule inhibitors deployed by Golly in the tumor pipeline are significantly different from the current popular PD-1/PD-L1 monoclonal antibodies, which are expected to overcome the strong immunogenicity of PD-1/PD-L1 monoclonal antibodies , The side effects are large; the monoclonal antibody preparation, transportation, storage cost is high, and the intravenous administration method is poor in accessibility and other limitations.
This differentiation determines that once the oral PD-L1 small molecule inhibitor is successfully developed, not only will it not lose its pricing power due to fierce homogenization competition, but it will have a more flexible business strategy.
The world’s current rapid progress in the research and development of oral PD-L1 small molecule inhibitors is GS-4224 (Gilead) and INCB086550 (Incyte), both of which are currently in the phase I clinical stage.
It is reported that the two oral PDs in the product pipeline -L1 small molecule inhibitor is about to complete preclinical research and start to declare IND.
In short, FASN inhibitors provide a new therapy for tumor lipid metabolism, and oral PD-L1 small molecule inhibitors are expected to further improve the efficacy, tolerance and compliance of immunotherapy.
Preclinical studies suggest that FASN has an impact on the tumor microenvironment, which provides a theoretical basis for FASN inhibitors combined with PD-1/PD-L1 inhibitors.
The layout of Golly Pharma’s clinical trials of FASN inhibitors and the development of oral PD-L1 small molecule inhibitors will provide more hope for treatment of cancer patients in the world.
Tumor lipid metabolism and oral checkpoint drugs
(source:internet, reference only)
Disclaimer of medicaltrend.org



