2021 AACR Annual Meeting: Focus on four new immunotherapies!
- Aspirin: Study Finds Greater Benefits for These Colorectal Cancer Patients
- Cancer Can Occur Without Genetic Mutations?
- Statins Lower Blood Lipids: How Long is a Course?
- Warning: Smartwatch Blood Sugar Measurement Deemed Dangerous
- Mifepristone: A Safe and Effective Abortion Option Amidst Controversy
- Asbestos Detected in Buildings Damaged in Ukraine: Analyzed by Japanese Company
2021 AACR Annual Meeting: Focus on four new immunotherapies!
- Israel new drug for COVID-19: EXO-CD24 can reduce deaths by 50%
- COVID-19 vaccines for children under 12 will be available soon
- Breakthrough infection of Delta: No difference from regular COVID-19 cases
- French research: ADE occurred in Delta variant and many doubts on it
- The viral load of Delta variant is 1260 times the original COVID-19 strain
2021 AACR Annual Meeting: Focus on four new immunotherapies!. 2021 AACR Annual Meeting: Focus on four new immunotherapies! CAR-T, TILs, cancer vaccines, and oncolytic viruses shine!
In the past few decades, the emergence of immunotherapy has made the medical community believe for the first time that it is possible to “cure” cancer, or that in the future we can control cancer as a chronic disease like hypertension and diabetes.
At the ongoing AACR event from April 10th to 15th, 2021, many new therapies have appeared one after another. The data is exciting, let us take a look at the latest progress of immunotherapy!
2021AACR: Focus on four new immunotherapies!
Immunotherapy is a wide range of cancer therapies, including cell-based therapies, vaccines, and immune system modulators. (At present, the five CAR-T therapies approved by the FDA can achieve an overall remission rate of more than 70% for specific hematological tumor types. These treatment methods have been proven to induce significant therapeutic responses-even in advanced stages with a survival period of only a few months. Cancer patients are also expected to be in complete remission, and in some cases a strong response lasts for months or even years).
The medical community hopes that this type of immunotherapy that relies on the autoimmune system to attack cancer can produce miracles of “curing” in more cancer patients. The four new types of immunotherapies on the AACR shine, bringing new hope to cancer patients.
01 The complete remission rate is 80%! The dawn of a new dual-specific CAR-T
As we all know, the results of CAR-T in the treatment of malignant hematological tumors are obvious to all. This is all due to the ancestral target of hematological tumors-CD19, which exists only in tumor cells and not in normal cells. In the cell. Therefore, this target can be used to lead CAR-T cells to find and destroy cancer cells in tumor treatment.
Currently, the United States has approved four CAR-T therapies for certain lymphomas, and recently approved the first CAR-T therapy for multiple myeloma. To
At the AACR conference in 2021, Dr. Sanaz Ghafouri, a researcher in hematology and oncology at UCLA Medical Center, announced a new research result that caused a huge sensation.
They developed a new type of bispecific CAR-T cell that can target two tumor antigens CD19/CD20 at the same time. This is the first bispecific CAR-T cell therapy developed and tested on patients using primitive memory T cells. This method may increase the persistence and expansion of CAR-T cells in patients, while avoiding Recurrence caused by the loss of tumor antigens.
This trial enrolled 5 patients with B-cell malignancies with positive expression of both CD19 and CD20 tumor antigens. These patients received an average of 4 therapies before. Naive memory T cells are extracted from each patient, engineered to express anti-CD19/CD20 CAR, amplified and then injected back into the patient.
After a median follow-up of 13 months, 4 out of 5 patients had a complete remission (80%)!
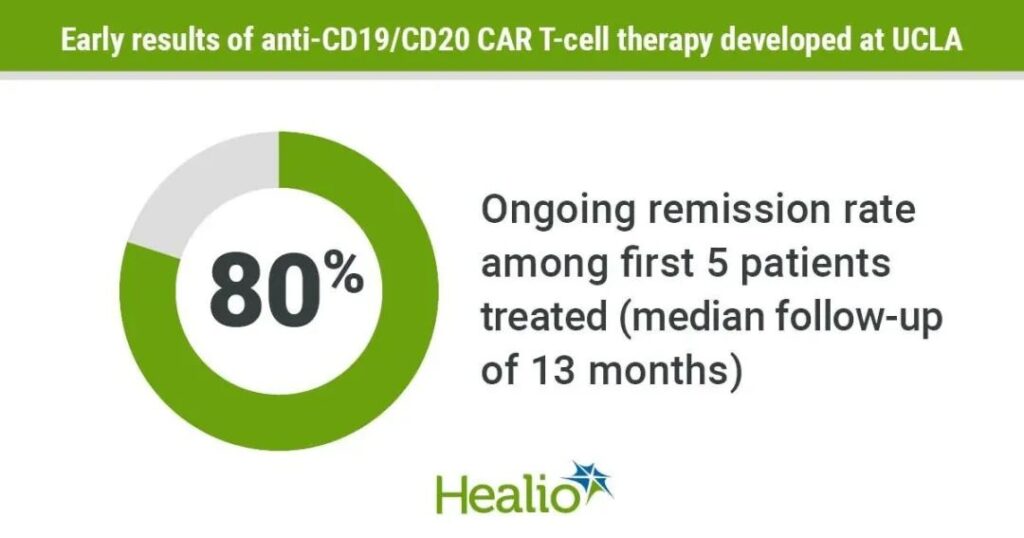
It is worth mentioning that there was no dose-limiting toxicity or neurotoxicity in any patient. All patients experience grade 1 cytokine release syndrome. Therefore, this new type of CAR-T therapy has excellent safety and provides the possibility of long-term sustained relief for this idiopathic disease!
02 The disease control rate is as high as 80.3%! TILs therapy is the first to declare war on solid tumors and is expected to be launched!
The most promising TILs (tumor infiltrating lymphocyte) therapy in solid tumors has been good news in the past two years, and has achieved amazing clinical results for a variety of clinically refractory solid tumors.
This new immunotherapy developed by the team of Professor Rosenberg, a leader in the immunology of the National Cancer Institute, has confirmed that TILs treatment in melanoma has the potential of a complete response (CR) that lasts for decades. This long-term The effect is attributed to the persistence of memory T cells. At this year’s AACR meeting, the innovative therapy LN-144 (Lifileucel) based on TILs cells updated the clinical results of melanoma and caused a sensation again.
C-144-01 is a phase 2 clinical trial that recruits 66 patients who have been diagnosed with stage IIIc or stage IV metastatic melanoma. At least 3 to 4 systemic treatments have failed. All of them have failed. 80% of the patients had received PD-1 inhibitor treatment, 80% had received CTLA-4 inhibitor, 23% had received BRAF/MEK inhibitor, and 44% had liver or brain metastases.
After these patients received surgery, the TILs cells in the tumor tissues were cultured and then reinfused. The average TIL cells injected: 28 x 10^9; IL-2 dose: 6 times.
In the newly reported trial of 66 patients who have received PD-1 for the treatment of advanced melanoma, the results show:
The disease control rate (DCR) is as high as 80.3%;
The objective remission rate reached 36.4% (complete remission was 4.5%, partial remission was 31.8%);
The median follow-up time was 8.8 months, which did not reach the median duration of response.
More strikingly, patients with PD-L1 negative also responded, indicating that patients who are ineffective against immune checkpoint inhibitors can still benefit from TILs therapy.
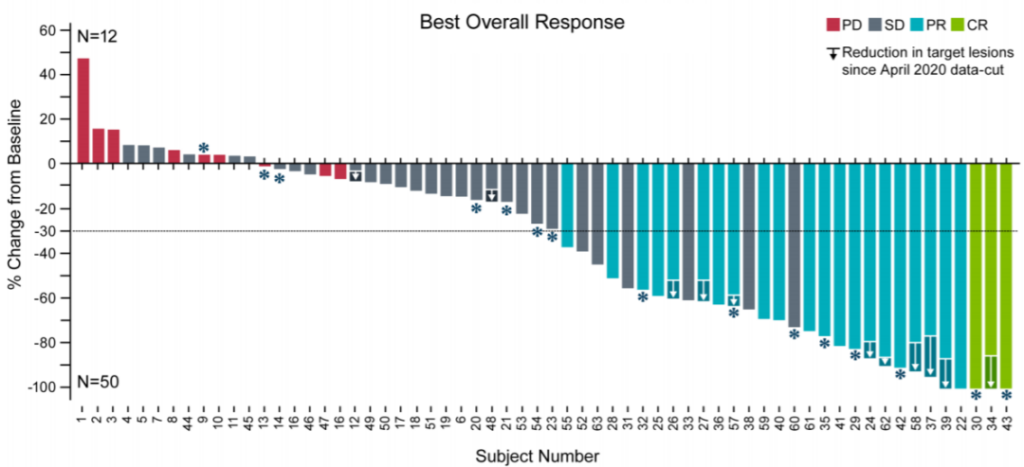
A patient with advanced melanoma had extensive tumor metastasis before treatment. One month after receiving TILs therapy, the focus was significantly reduced, and complete remission was achieved within 6 months of treatment. After two years of treatment, it was still in complete remission, and tumor reactivity persisted CD8+ T cells.
Currently, TILs therapy is expected to submit a marketing application in 2021. If approved by the FDA, this will be the first cellular immunotherapy for solid tumors and will bring huge survival benefits to cancer patients.
03 Personalized cancer vaccine is expected to treat a variety of cancers
After ending smallpox, polio, and completely stifling the COVID-19 pneumonia that is raging around the world, researchers hope to use vaccines to treat cancer. Is this possible? At the AACR conference in 2021, a new type of personalized cancer vaccine makes this vision a reality.
Different from traditional cancer vaccines, the personalized cancer vaccine (PGV-001) is an adjuvant therapy vaccine specially used after standard treatment, which helps to remove residual cancer cells in the body and prolong the survival of patients.
After receiving surgery and standard treatment, 13 patients with various types of cancer received 7-10 doses of personalized PGV-001 vaccine and immunostimulant poly-ICLC. After an average follow-up of 880 days (over 2 years), 4 patients showed no signs of cancer, 4 patients were receiving follow-up treatment, 3 patients died, and 2 patients lost follow-up. The median progression-free survival was 618 days. The vaccine is well tolerated, and only grade 1 injection site reactions have occurred in approximately half of the patients.
According to preliminary clinical trial results, the vaccine can potentially be used to treat many types of tumors.
04 Oncolytic virus G207 is expected to fight deadly brain tumors!
Oncolytic virus is a type of virus that can effectively infect and kill cancer cells. At this AACR conference, the results of a phase I clinical trial of a new oncolytic virus therapy were announced, which is very exciting.
The new oncolytic virus uses a modified herpes simplex virus type 1 (HSV-1, a common cause of cold sores), which can infect tumor cells but not normal cells. This oncolytic virus is called G207. Since HSV-1 naturally infects cells in the surrounding and central nervous system, it may be ideal for brain tumors.
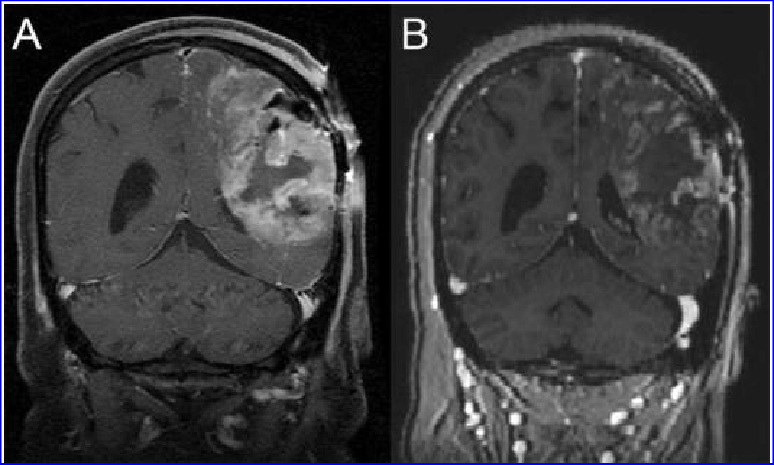
Comparison of glioblastoma before and after G207 treatment. (A) Before treatment (B) One month after G207 inoculation, it shows a clear response to G207.
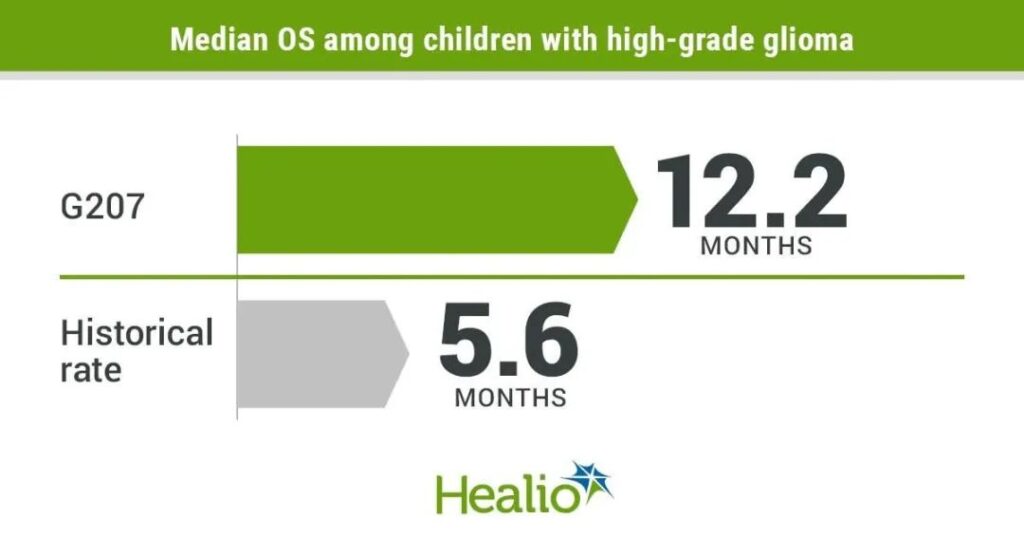
The trial included 12 patients with high-grade glioma aged 7 to 18, all of whom had advanced after standard treatment. After receiving the G207 virus injection, some patients also receive a single small dose of radiation to enhance virus replication and spread throughout the tumor. The results showed that a response was observed in 11 patients, with a median overall survival of 12.2 months, a 120% increase over the typical overall survival of progressive childhood high-grade glioma.
G207 used alone or in combination with radiation therapy is well tolerated, has no dose-limiting toxicity, treatment-related grade 3/4 adverse events, and no evidence of virus penetration into blood, saliva, or conjunctiva.
In addition, the analysis of tumor tissues before and after treatment showed that the number of tumor-infiltrating immune cells, including CD4+ and CD8+ T cells, increased within three to nine months after G207 infusion. T cell infiltration was also observed a few centimeters from the G207 inoculation site.
These results indicate that this treatment method can transform immune “cold” pediatric high-grade gliomas with few immune cells into “hot” tumors with abundant immune cells, which will be an effective research direction for children’s immunotherapy.
Concluding remarks
Immunotherapy is hailed as the hope of overcoming cancer. Whether it is CAR-T therapy, immune checkpoint inhibitor therapy, and cancer vaccines, they are just the tip of the iceberg of immunology, and researchers are digging for more information contained in the iceberg.
Expect more breakthroughs in immunotherapy and bring more miracles to cancer patients.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



