Nature: Pregnancy will reprogram breast cells and reduce the risk of breast cancer
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Nature: Pregnancy will reprogram breast cells and reduce the risk of breast cancer
Nature: Pregnancy will reprogram breast cells and reduce the risk of breast cancer. This study provides new insights into the role of pregnancy in changing the epigenome landscape and inhibiting the malignant transformation of breast epithelial cells.
It also shows that pregnancy reduces breast cancer risk at least in part through epigenome reprogramming.

In January 2021, the World Health Organization’s International Agency for Research on Cancer (IARC) issued the latest global cancer burden data in 2020. The latest morbidity, mortality, and cancer development trends of 36 cancer types in 185 countries and regions around the world are estimated. Details: The latest global cancer data for 2020.
One of the most obvious changes in the latest global cancer burden data in 2020 is that the number of new cases of breast cancer has increased rapidly, replacing lung cancer with the number of new cases of 2.26 million as the world’s largest cancer.
One of the fundamental reasons for the increase in the incidence of breast cancer is the continuous changes in breast cancer risk factors, such as postponed births and fewer births. This is most obvious in countries undergoing social and economic transition. Overweight and obesity, and lack of exercise are also The reason for the increasing incidence of breast cancer worldwide.
Previous studies have shown that women who become pregnant at the age of 25 and before can reduce the overall risk of breast cancer by more than 30%. However, the molecular mechanism behind this protective effect is still unclear.
Researchers from the Cold Spring Harbor Laboratory in the United States published a research paper titled “Pregnancy reprograms the epigenome of mammary epithelial cells and blocks the development of premalignant lesions” in Nature Communications.
The research shows thatPregnancy can lead to epigenetic reprogramming of breast epithelial cells, resulting in the failure of the strong oncogene c-MYC as a transcription factor to bind and exert carcinogenic effects.
This study provides new insights into the role of pregnancy in changing the epigenome landscape and inhibiting the malignant transformation of breast epithelial cells. It also shows that pregnancy reduces breast cancer risk at least in part through epigenome reprogramming.
These findings also provide new directions for future cancer treatments and provide a better way to identify cancer risks before tumors develop.


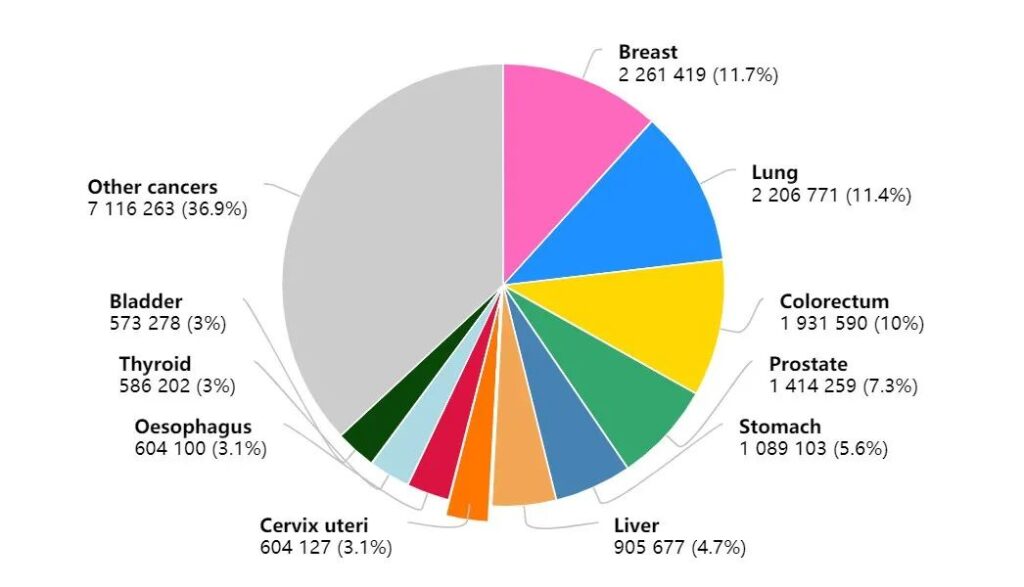
Among the top ten cancer types in the number of new cancer cases in 2020, breast cancer tops the list
In mammals and humans, the physiological stimulation brought about by pregnancy can lead to considerable developmental reorganization, which plays a key role in breast lactation and the nurturing of offspring.
The function of pregnancy signals in mammary epithelial cells (MEC) is not only to induce milk production and lactation, but also to change the risk of breast cancer in animals and humans.
After 45 years of age is the high incidence of breast cancer, and women who are pregnant and give birth before the age of 30 can reduce the risk of breast cancer in the long-term. Pregnancy before the age of 25 can reduce the overall risk of breast cancer by more than 30%.
Previous studies on animal models have also shown that pregnancy or treatment with progesterone can inhibit breast cancer or hereditary breast cancer caused by carcinogens.
Pregnancy can also cause a series of cellular and molecular changes in the breast epithelial cells (MEC) of adult women. Previous studies have shown that pregnancy can induce specific changes in DNA methylation in breast epithelial cells, which suggests that the inhibitory effect of pregnancy on breast cancer may be through epigenetics.
To evaluate the above hypothesis, the research team characterized gene activation and enhancer tissue dynamics in mouse mammary epithelial cells because they respond to pregnancy signals or early tumor development.
In order to further characterize the effect of pregnancy-induced epigenomic changes on the oncogene expression response, the research team used the CAGMYC transgenic mouse model, which overexpresses the oncogene c-MYC, which can be induced by doxycycline (DOX) Developed into breast cancer.
The c-MYC gene is overexpressed in more than 50% of breast cancers, and plays a carcinogenic effect by regulating the expression of other genes as a transcription factor.
Experimental results show. Mammary epithelial cells (MEC) after pregnancy did not undergo malignant transformation in response to the overexpression of the oncogene c-MYC under in vivo or in vitro conditions. Further transcriptomics and epigenetic analysis showed that c-MYC overexpression drives MEC after pregnancy to
Senescence-like state, which is the moment in the cell life cycle between dying, survival, and potential cancer.
For this discovery, the corresponding author, Dr. Camila dos Santos, used an image metaphor to explain that the way the pregnancy itself changes the DNA region can be imagined as a “yo-yo”, and the center of this “yo-yo” is the nucleosome (Nucleosome), a nucleosome is a bunch of proteins that protect DNA.
When you release the “Yo-Yo”, it’s like a part of DNA is opened, and transcription factors can bind to it to turn on or off gene expression; when you take the “Yo-Yo” back, it’s like these DNAs will come back. Closed state, at this time transcription factors cannot bind to it. Pregnancy uses this model to prevent the oncogene c-MYC, which is a transcription factor, from being combined, so that it cannot play a carcinogenic effect.
Camila dos Santos and her team are studying human breast tissue organoids to understand whether human breast tissue behaves like mouse tissue.
She also transplanted breast cells altered by pregnancy into mice that had never been pregnant to determine whether the altered cells would affect the environment of non-pregnancy. These experiments have proposed new drug targets for breast cancer treatment.
These findings have opened the door for us to further explore issues that have not been explored before.
This study provides new insights into the role of pregnancy in changing the epigenome landscape and inhibiting the malignant transformation of breast epithelial cells. It also shows that pregnancy reduces breast cancer risk at least in part through epigenome reprogramming.
These findings also provide new directions for future cancer treatments and provide a better way to identify cancer risks before tumors develop.
(source:internet, reference only)
Disclaimer of medicaltrend.org



