Chemotherapy + CAR-T treatment for high tumor burden lymphoma
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Chemotherapy + CAR-T treatment to improve the remission rate and overall survival rate of patients with high tumor burden lymphoma
Chemotherapy + CAR-T treatment for high tumor burden lymphoma. The treatment response and prognosis of refractory/relapsed (R/R) diffuse large B-cell lymphoma (DLBCL) are poor. The current standard treatment includes high-dose chemotherapy followed by autologous stem cell transplantation. However, the long-term remission rate associated with this type of treatment is only 10-20%.
Since 2017, there have been 5 CAR-T cell therapies on the market, and CAR-T therapies have shown significant effects in B-cell malignancies. Previous clinical trials have shown that its objective remission rate for B-cell lymphoma is 50-80%, and its complete remission rate is 30-50%. The NCCN guidelines also recommend CAR-T therapy for patients with diffuse large B-cell lymphoma (DLBCL) who have achieved partial remission after second-line treatment and patients who have relapsed or progressed after complete remission after second-line treatment.
CAR-T therapy is usually associated with potentially fatal toxicities, including cytokine release syndrome (CRS) and neurotoxicity, especially in the case of high tumor burden. In addition, high tumor metabolic volume has been identified as a predictor of early progression of diffuse large B-cell lymphoma (DLBCL) in CAR-T treatment. Therefore, prior to the start of CAR-T treatment, intensive chemotherapy can reduce tumor burden and improve the effect of CAR-T treatment.
Recently, the team of Chief Physician Deng Qi of the First Central Hospital Affiliated to Nankai University published a title in Frontiers in Oncology (IF=6.244): Intensive Debulking Chemotherapy Improves the Short-Term and Long-Term Efficacy of Anti-CD19-CAR- Clinical research paper of T in Refractory/Relapsed DLBCL With High Tumor Bulk.

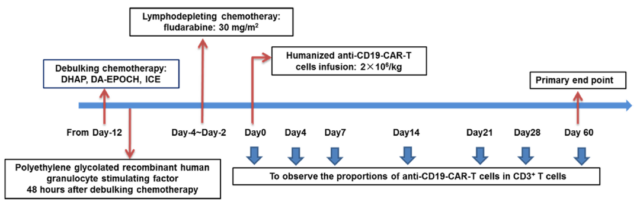
The team has previously demonstrated that CAR-T therapy in refractory/relapsed B-cell acute lymphoblastic leukemia (B-ALL) is related to high treatment response and controllable toxicity after enhanced lymphocyte depletion chemotherapy.
Intensive chemotherapy is often used in the treatment of refractory/relapsed (R/R) diffuse large B-cell lymphoma (DLBCL), such as DHAP (dexamethasone, cisplatin, and cytarabine), DA-EPOCH (dose) Adjusted etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin), ICE (ifosfamide, carboplatin, and etoposide), and GemOx (gemcitabine and oxaliplatin).
Since many patients with refractory/relapsed (R/R) diffuse large B-cell lymphoma (DLBCL) with large tumors can temporarily reduce their tumor burden after intensive chemotherapy, the research team speculates that reducing the patient’s tumor burden can Improve the effect of anti-CD19-CAR-T cell therapy.
The research team recruited 57 patients with refractory/relapsed (R/R) diffuse large B-cell lymphoma (DLBCL) to participate in this clinical trial, of which 15 patients with high tumor burden received only cytoreductive chemotherapy. The remaining 42 patients received CAR-T cell therapy (17 patients with low tumor burden were directly treated with CAR-T, and they served as a control group).
Twenty-five patients with high tumor burden received tumor-reducing chemotherapy followed by lymphocyte depletion chemotherapy. Among them, 19 were sensitive to chemotherapy and 6 were resistant to chemotherapy. Then received humanized anti-CD19-CAR-T cell therapy.
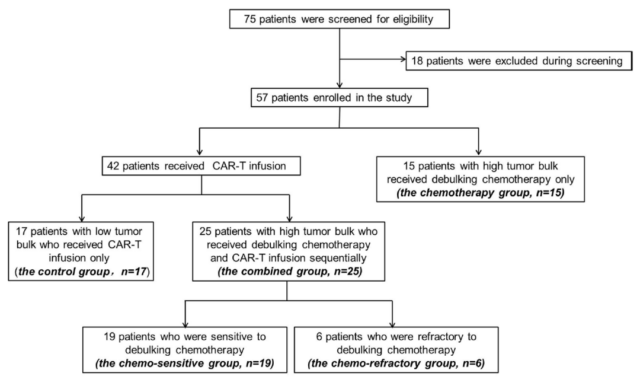
All patients were evaluated within 2 months after anti-CD19-CAR-T cell infusion. Of the 19 patients in the chemotherapy-sensitive group, 18 had an objective response (objective response rate of 95.0%), and 13 had a complete response (complete response rate of 68.4%). Of the 6 patients in the chemotherapy resistance group, 3 had objective remission (objective remission rate was 50.0%), of which 3 had partial remission and 3 had disease progression.
As a control group, the objective remission rate of 17 patients with low tumor burden who directly underwent CAR-T cell therapy was 88%. The complete remission rate was 59%.
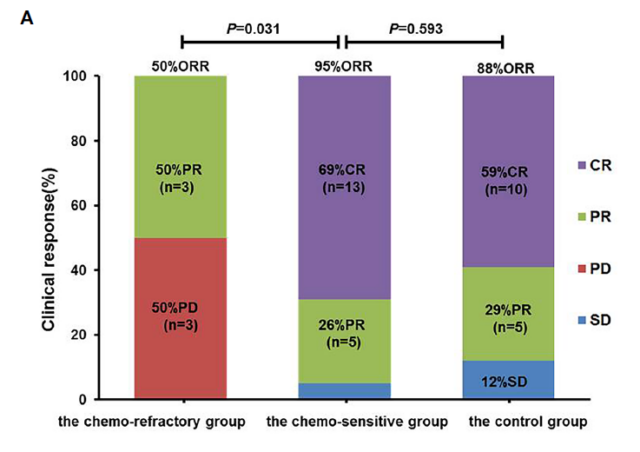
Among the groups receiving CAR-T therapy, there was no significant difference in the probability of cytokine release syndrome (CRS), no patients had neurological toxicity, and no patients died of cytokine release syndrome and neurotoxicity.
The 1-year progression-free survival rate and overall survival rate of the 19 patients who received CAR-T treatment who were sensitive to chemotherapy were 52.6% and 57.9%, respectively, which were higher than the 6 patients who received CAR-T treatment who were resistant to chemotherapy , And also higher than 15 patients with high tumor burden who only received chemotherapy for tumor reduction. This also shows that for patients with high tumor burden refractory/relapsed (R/R) diffuse large B-cell lymphoma (DLBCL), chemotherapy alone does not improve the prognosis.
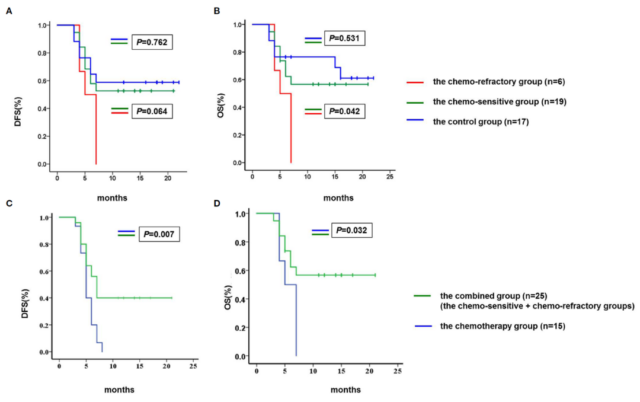
Overall, this clinical trial showed that effective chemotherapy for tumor reduction improved the objective response rate and overall survival rate of patients with high tumor burden, making it comparable to the results of patients with low tumor burden. This also provides a new treatment option for the treatment of patients with refractory/relapsed (R/R) diffuse large B-cell lymphoma (DLBCL) with high tumor burden—enhanced chemotherapy for tumor reduction and CAR-T cells Combination therapy.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



