Colorectal cancer: Is there still no targeted drug for KRAS mutation?
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Colorectal cancer: Is there still no targeted drug for KRAS mutation?
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Colorectal cancer: Is there still no targeted drug for KRAS mutation?
KRAS is a common mutated gene in colorectal cancer (CRC), but it has long faced the dilemma of “targets without drugs”. Clinical research data supports the combination of MEK inhibitors and PI3K inhibitors in the treatment of advanced CRC to improve the efficacy and avoid drug resistance.
There are also studies exploring the effects of immunotherapy combined with these targeted inhibitors. A recent review published by AACR summarizes current research on RAS CRC.
Background
KRAS mutation (mt KRAS) is one of the most important proto-oncogene mutations known so far, and the incidence in colorectal cancer (CRC) is about 60%. Current clinical trial data provide support for the use of MEK inhibitors in combination with any PI3K inhibitor in patients with metastatic CRC. This program can avoid the development of drug resistance and has better efficacy than monotherapy.
For patients with limited options for subsequent treatment, there are also clinical studies evaluating the efficacy of immunotherapy combined with inhibitors of different pathways. This review discusses methods and clinical studies for the direct or indirect treatment of KRAS mutations.
Targeted therapy directly against KRAS
The surface of KRAS is relatively smooth, making it difficult to bind small molecule drugs. In recent years, new insights into the structure and function of KRAS protein have revealed the possibility of targeted alternative activation of Ras. In 2013, Ostrem and colleagues discovered for the first time a compound that selectively targets the 12-cysteine in the KRAS G12C mutation without inhibiting wild-type KRAS.
These compounds lock mt KRAS G12C in its inactive GDP binding state by binding to the pocket near the nucleotide binding site and preventing GTP from repolarizing. Similar direct targeting methods have prompted the development of adagrasib, ARS-1620, ARS-853, SML-8-73-1 and sotorasib, which have good therapeutic potential as specific inhibitors of KRAS G12C alleles. Related clinical studies are shown in Table 1.
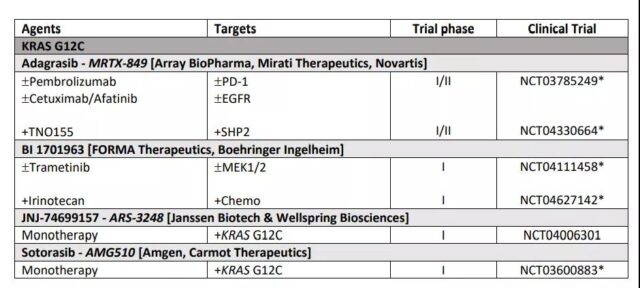
Table 1 Clinical studies of KRAS mutation targeted therapy for colorectal cancer
Indirect treatment for KRAS
PI3K inhibitor
It mainly includes three categories: selective isomers, pan-PI3K inhibitors and dual pan-PI3K inhibitors.
Many PI3K selective isoforms (p110α, β, δ, γ) inhibitors are in clinical studies for the treatment of advanced solid tumors, hematological malignancies and lymphomas. Due to the restricted expression of p110δ and p110γ in blood cells, compared with solid tumors, e PI3Kδ and PI3Kγ selective isoform inhibitors are more effective in malignant tumors of the blood system.
Recent studies have shown that inhibition of p110α or p110β can lead to reactivation of PI3K signaling, thus limiting the clinical efficacy of the drug. These evidences support that pan-PI3K inhibitors or the combination of PI3Kα and PI3Kβ can better inhibit the PI3K pathway. Pan-PI3K inhibitors can target four isoforms of type I PI3K kinase (α, β, 214 δ, γ).
The main drawback of this type of drug is a wide range of drug-related adverse reactions, including anorexia, fatigue, nausea, and hyperglycemia, which limit the dosage of the drug. Dual PI3K-mTOR inhibitors can more effectively shut down the PI3K-AKT-mTOR pathway and cause tumor regression. Although the preclinical data and in vitro experiments of dual PI3K-mTOR inhibitors have shown good tumor inhibition effects, the clinical effects are not very satisfactory.
AKT inhibitor
Most AKT inhibitors block AKT 1, 2, 3 by interfering with ATP or partially binding to the ATP binding site. Although the targeting selectivity of AKT is expected to be higher than that of PI3K inhibitors, the clinical results are not satisfactory. Side effects of AKT inhibitors include severe skin rash and high blood sugar.
Some drugs did not show clinical benefit in phase II/III studies. Two phase II clinical trials of Enzastaurin for colorectal cancer (NCT00192114 and NCT00437268) have been completed, and the results are yet to be announced.
- Capivasertib, developed by AstraZeneca, has been shown to inhibit all AKT isoforms and AGC family kinases in in vivo experiments.
- Davies and colleagues reported that wt-RAS, PI3KCA, or PTEN mutant cell lines are sensitive to capivasertib. The phase I trial of Capivasertib in solid tumor patients (NCT01353781) showed that intermittent administration has anti-tumor activity, and the phase II combination therapy study (NCT02576444) is currently underway.
mTOR inhibitor
Most studies of mTOR inhibitor monotherapy in patients with mCRC have not shown clinical benefit. It may be related to drug resistance caused by elF4E binding protein 1 kinase. Combining upstream receptor inhibitors has become the focus of research.
Phase I/II clinical studies have shown that everolimus combined with modified FOLFOX-6+bevacizumab first-line treatment of mCRC patients, 96% of patients PFS more than 6 months (NCT01047293). A multicenter phase II study (NCT01058655) showed that everolimus combined with another type of VEGF inhibitor tivozanib is well tolerated. Everolimus combined with irinotecan and cetuximab have shown benefits for patients with relapsed KRAS wild-type mCRC (NCT00478634 & NCT00522665).
RAF inhibitor
The first-generation BRAF inhibitor targets the BRAF kinase domain, including vemurafenib, dabrafenib, encorafenib, and sorafenib, which have been tried on BRAFV600E mCRC patients.
- Vemurafenib is not effective as a single agent, and combined treatment can improve the efficacy. Shanghai Changzheng Hospital is currently recruiting advanced BRAFV600E mCRC patients to evaluate the efficacy of vemurafenib+cetuximab+FOLFIRI (NCT03727763).
- Dabrafenib combined with Pertuzumab and MEK1/2 inhibitor trametinib showed anti-tumor activity.
- Encorafenib is undergoing multiple phase III studies, including nivolumab, cetuximab, MEK inhibitor (binimetinib), PI3Ka inhibitor (alpelisib), cytotoxic drugs (5-FU, bevacizumab) , Capecitabine, irinotecan, leucovorin, oxaliplatin) combination therapy, promising prospects.
MEK inhibitor
The most promising MEK inhibitors are binimetinib and trametinib, and phase II/III clinical studies are ongoing.
ERK inhibitor RAF and MEK drug resistance is related to the reactivation of ERK1/2, so blocking ERK1/2 can overcome the resistance problem of upstream RAF and MEK inhibitors. However, it is still unclear whether the efficacy of ERK inhibitors is better than MEK inhibitors.
Immune checkpoint inhibitors (ICIs)
The clinical benefit of ICIs in CRC is mainly limited to patients with highly unstable microsatellites (MSI/dMMR). This subgroup accounts for approximately 15% of all CRCs. MSI-H CRC is characterized by a large number of tumor infiltrating lymphocytes (TILs) and high immunogenicity in the tumor microenvironment. It is worth noting that MSI-H patients have higher expression levels of PD-1, PD-L1 and CTLA-4.
Therefore, blocking PD-1/PD-L1 and CD80/CTLA4 by ICIs can enhance T cell activation and promote tumor cell killing. Preclinical experiments reported the synergistic therapeutic effect of PD-1 or PD-L1 antibody combined with MEK inhibitor. Animal experiments show that this combination therapy significantly inhibits tumor growth and increases CD4+ and CD8+ T cells in the tumor.
In 2018, ipilimumab combined with nivolumab or nivolumab was approved for use in MSI-H mCRC. Phase II NICHE and CheckMate142 studies are currently underway. The study mainly evaluates ipilimumab, nivolumab combined with COX2 inhibitor celecoxib, MEK inhibitor cobimetinib, anti-CD38 antibody daratumumab, or anti-LAG-3 antibody BMS 986016 for CRC The curative effect (NCT03026140, NCT02060188). At the same time, studies have reported that single-dose ipilimumab combined with double-dose nivolumab is effective for patients with dMMR.
Recently, with studies on NCT03785249, NCT03475004, NCT03374254, NCT02563002, etc., Pembrolizumab was approved for use in MSI mCRC. Le and colleagues found that MMR status was correlated with the patient’s benefit of pembrolizumab (NCT01876511), and the PFS rate was 78%. Andre and Le’s research further supports pembrolizumab as a single-agent treatment of MSI-H CRC. But pembrolizumab combination therapy showed no clinical benefit for MMR CRC (NCT02981524). Another phase II study evaluated the efficacy of PD-L1 antibody Avelumab (NCT03150706) on MSI-H or POLE mutant mCRC. Atezolizumab monoclonal antibody treatment of dMMR/MSI-H mCRC has a small number of studies (NCT02997228, NCT03866239, NCT02912559).
So far, ICIs have been very successful in the subgroup of dMMR/MSI-H patients, but they are not sensitive to MMR CRC patients. Further efforts are needed to determine which subgroups of patients can benefit from ICI combination therapy. It is expected that ICI will become the standard treatment plan for dMMR mCRC as soon as possible.
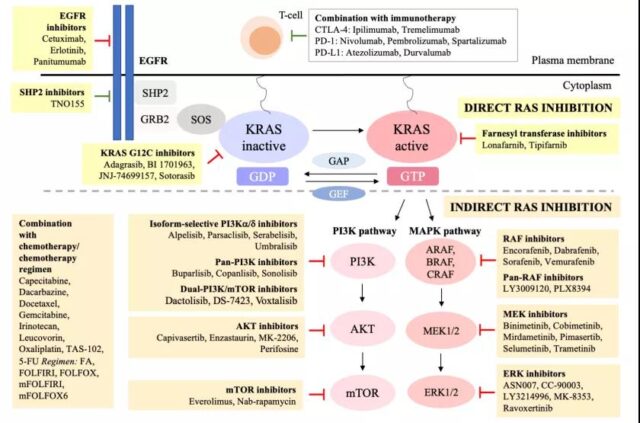
Figure 1 Schematic diagram of KRAS targeted therapy for colorectal cancer (Source: Reference 1)
Sum up:
In short, with the advancement of molecular therapy, the treatment of CRC has made great progress. Early attention focused on the development of upstream PI3K and RAF inhibitors to block the activation of mTOR and ERK. Later, it was discovered that RAS tumor cells can reactivate mTOR and ERK through dynamic reorganization of downstream signaling networks, leading to resistance to upstream inhibitors.
Different molecular subtypes will significantly affect the prognosis and treatment strategies of CRC. This means that future clinical studies of CRC need to consider stratification factors such as multi-drug resistance mechanisms, different molecular subtypes, patient prognosis, and biomarkers. At present, a number of clinical studies on combined therapy of targeted inhibitors and immune checkpoint inhibitors are underway.
Colorectal cancer: Is there still no targeted drug for KRAS mutation?
KRAS is a common mutated gene in colorectal cancer (CRC), but it has long faced the dilemma of “targets without drugs”. Clinical research data supports the combination of MEK inhibitors and PI3K inhibitors in the treatment of advanced CRC to improve the efficacy and avoid drug resistance.
There are also studies exploring the effects of immunotherapy combined with these targeted inhibitors. A recent review published by AACR summarizes current research on RAS CRC.
Background
KRAS mutation (mt KRAS) is one of the most important proto-oncogene mutations known so far, and the incidence in colorectal cancer (CRC) is about 60%. Current clinical trial data provide support for the use of MEK inhibitors in combination with any PI3K inhibitor in patients with metastatic CRC. This program can avoid the development of drug resistance and has better efficacy than monotherapy.
For patients with limited options for subsequent treatment, there are also clinical studies evaluating the efficacy of immunotherapy combined with inhibitors of different pathways. This review discusses methods and clinical studies for the direct or indirect treatment of KRAS mutations.
Targeted therapy directly against KRAS
The surface of KRAS is relatively smooth, making it difficult to bind small molecule drugs. In recent years, new insights into the structure and function of KRAS protein have revealed the possibility of targeted alternative activation of Ras. In 2013, Ostrem and colleagues discovered for the first time a compound that selectively targets the 12-cysteine in the KRAS G12C mutation without inhibiting wild-type KRAS.
These compounds lock mt KRAS G12C in its inactive GDP binding state by binding to the pocket near the nucleotide binding site and preventing GTP from repolarizing. Similar direct targeting methods have prompted the development of adagrasib, ARS-1620, ARS-853, SML-8-73-1 and sotorasib, which have good therapeutic potential as specific inhibitors of KRAS G12C alleles. Related clinical studies are shown in Table 1.

Table 1 Clinical studies of KRAS mutation targeted therapy for colorectal cancer
Indirect treatment for KRAS
PI3K inhibitor
It mainly includes three categories: selective isomers, pan-PI3K inhibitors and dual pan-PI3K inhibitors.
Many PI3K selective isoforms (p110α, β, δ, γ) inhibitors are in clinical studies for the treatment of advanced solid tumors, hematological malignancies and lymphomas. Due to the restricted expression of p110δ and p110γ in blood cells, compared with solid tumors, e PI3Kδ and PI3Kγ selective isoform inhibitors are more effective in malignant tumors of the blood system.
Recent studies have shown that inhibition of p110α or p110β can lead to reactivation of PI3K signaling, thus limiting the clinical efficacy of the drug. These evidences support that pan-PI3K inhibitors or the combination of PI3Kα and PI3Kβ can better inhibit the PI3K pathway. Pan-PI3K inhibitors can target four isoforms of type I PI3K kinase (α, β, 214 δ, γ).
The main drawback of this type of drug is a wide range of drug-related adverse reactions, including anorexia, fatigue, nausea, and hyperglycemia, which limit the dosage of the drug. Dual PI3K-mTOR inhibitors can more effectively shut down the PI3K-AKT-mTOR pathway and cause tumor regression. Although the preclinical data and in vitro experiments of dual PI3K-mTOR inhibitors have shown good tumor inhibition effects, the clinical effects are not very satisfactory.
AKT inhibitor
Most AKT inhibitors block AKT 1, 2, 3 by interfering with ATP or partially binding to the ATP binding site. Although the targeting selectivity of AKT is expected to be higher than that of PI3K inhibitors, the clinical results are not satisfactory. Side effects of AKT inhibitors include severe skin rash and high blood sugar.
Some drugs did not show clinical benefit in phase II/III studies. Two phase II clinical trials of Enzastaurin for colorectal cancer (NCT00192114 and NCT00437268) have been completed, and the results are yet to be announced.
- Capivasertib, developed by AstraZeneca, has been shown to inhibit all AKT isoforms and AGC family kinases in in vivo experiments.
- Davies and colleagues reported that wt-RAS, PI3KCA, or PTEN mutant cell lines are sensitive to capivasertib. The phase I trial of Capivasertib in solid tumor patients (NCT01353781) showed that intermittent administration has anti-tumor activity, and the phase II combination therapy study (NCT02576444) is currently underway.
mTOR inhibitor
Most studies of mTOR inhibitor monotherapy in patients with mCRC have not shown clinical benefit. It may be related to drug resistance caused by elF4E binding protein 1 kinase. Combining upstream receptor inhibitors has become the focus of research.
Phase I/II clinical studies have shown that everolimus combined with modified FOLFOX-6+bevacizumab first-line treatment of mCRC patients, 96% of patients PFS more than 6 months (NCT01047293). A multicenter phase II study (NCT01058655) showed that everolimus combined with another type of VEGF inhibitor tivozanib is well tolerated. Everolimus combined with irinotecan and cetuximab have shown benefits for patients with relapsed KRAS wild-type mCRC (NCT00478634 & NCT00522665).
RAF inhibitor
The first-generation BRAF inhibitor targets the BRAF kinase domain, including vemurafenib, dabrafenib, encorafenib, and sorafenib, which have been tried on BRAFV600E mCRC patients.
- Vemurafenib is not effective as a single agent, and combined treatment can improve the efficacy. Shanghai Changzheng Hospital is currently recruiting advanced BRAFV600E mCRC patients to evaluate the efficacy of vemurafenib+cetuximab+FOLFIRI (NCT03727763).
- Dabrafenib combined with Pertuzumab and MEK1/2 inhibitor trametinib showed anti-tumor activity.
- Encorafenib is undergoing multiple phase III studies, including nivolumab, cetuximab, MEK inhibitor (binimetinib), PI3Ka inhibitor (alpelisib), cytotoxic drugs (5-FU, bevacizumab) , Capecitabine, irinotecan, leucovorin, oxaliplatin) combination therapy, promising prospects.
MEK inhibitor
The most promising MEK inhibitors are binimetinib and trametinib, and phase II/III clinical studies are ongoing.
ERK inhibitor RAF and MEK drug resistance is related to the reactivation of ERK1/2, so blocking ERK1/2 can overcome the resistance problem of upstream RAF and MEK inhibitors. However, it is still unclear whether the efficacy of ERK inhibitors is better than MEK inhibitors.
Immune checkpoint inhibitors (ICIs)
The clinical benefit of ICIs in CRC is mainly limited to patients with highly unstable microsatellites (MSI/dMMR). This subgroup accounts for approximately 15% of all CRCs. MSI-H CRC is characterized by a large number of tumor infiltrating lymphocytes (TILs) and high immunogenicity in the tumor microenvironment. It is worth noting that MSI-H patients have higher expression levels of PD-1, PD-L1 and CTLA-4.
Therefore, blocking PD-1/PD-L1 and CD80/CTLA4 by ICIs can enhance T cell activation and promote tumor cell killing. Preclinical experiments reported the synergistic therapeutic effect of PD-1 or PD-L1 antibody combined with MEK inhibitor. Animal experiments show that this combination therapy significantly inhibits tumor growth and increases CD4+ and CD8+ T cells in the tumor.
In 2018, ipilimumab combined with nivolumab or nivolumab was approved for use in MSI-H mCRC. Phase II NICHE and CheckMate142 studies are currently underway. The study mainly evaluates ipilimumab, nivolumab combined with COX2 inhibitor celecoxib, MEK inhibitor cobimetinib, anti-CD38 antibody daratumumab, or anti-LAG-3 antibody BMS 986016 for CRC The curative effect (NCT03026140, NCT02060188). At the same time, studies have reported that single-dose ipilimumab combined with double-dose nivolumab is effective for patients with dMMR.
Recently, with studies on NCT03785249, NCT03475004, NCT03374254, NCT02563002, etc., Pembrolizumab was approved for use in MSI mCRC. Le and colleagues found that MMR status was correlated with the patient’s benefit of pembrolizumab (NCT01876511), and the PFS rate was 78%. Andre and Le’s research further supports pembrolizumab as a single-agent treatment of MSI-H CRC. But pembrolizumab combination therapy showed no clinical benefit for MMR CRC (NCT02981524). Another phase II study evaluated the efficacy of PD-L1 antibody Avelumab (NCT03150706) on MSI-H or POLE mutant mCRC. Atezolizumab monoclonal antibody treatment of dMMR/MSI-H mCRC has a small number of studies (NCT02997228, NCT03866239, NCT02912559).
So far, ICIs have been very successful in the subgroup of dMMR/MSI-H patients, but they are not sensitive to MMR CRC patients. Further efforts are needed to determine which subgroups of patients can benefit from ICI combination therapy. It is expected that ICI will become the standard treatment plan for dMMR mCRC as soon as possible.

Figure 1 Schematic diagram of KRAS targeted therapy for colorectal cancer (Source: Reference 1)
Sum up: (Colorectal cancer: Is there still no targeted drug for KRAS mutation?)
In short, with the advancement of molecular therapy, the treatment of CRC has made great progress. Early attention focused on the development of upstream PI3K and RAF inhibitors to block the activation of mTOR and ERK. Later, it was discovered that RAS tumor cells can reactivate mTOR and ERK through dynamic reorganization of downstream signaling networks, leading to resistance to upstream inhibitors.
Different molecular subtypes will significantly affect the prognosis and treatment strategies of CRC. This means that future clinical studies of CRC need to consider stratification factors such as multi-drug resistance mechanisms, different molecular subtypes, patient prognosis, and biomarkers. At present, a number of clinical studies on combined therapy of targeted inhibitors and immune checkpoint inhibitors are underway.
Colorectal cancer: Is there still no targeted drug for KRAS mutation?
Colorectal cancer: Is there still no targeted drug for KRAS mutation?
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



