Ketogenic diet vs. Calorie restriction diet: Which can limit tumor growth?
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Ketogenic diet vs. Calorie restriction diet: Which can limit tumor growth?
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Ketogenic diet vs. Calorie restriction diet: Which can limit tumor growth? Nature’s latest research gives this answer.
Every bite of food we eat deeply affects the health and function of the body. In recent years, there is some evidence that dietary intervention can help slow the growth of tumors.
On October 20, 2021, the team of Matthew G. Vander Heiden from Massachusetts Institute of Technology published a research paper titled Low glycaemic diets alter lipid metabolism to influence tumour growth in Nature, revealing how a diet affects The unique mechanism of cancer cells, that is, calorie restriction (ie calories) rather than a ketogenic diet can inhibit tumor growth in mouse models of pancreatic cancer.
Although both dietary interventions are thought to change tumor growth, the new study shows that calories Restriction can limit tumor growth by changing the lipid levels in the tumor.

source: Nature
Research Background
Diet intervention can change the level of metabolites in the tumor microenvironment, which may affect cancer cell metabolism and thus change tumor growth. Low-glycemic diets, such as calorie restriction and ketogenic diets, can minimize spikes in blood sugar and insulin levels. In some animal models, this effect is related to the inhibition of tumor growth. However, it is unclear whether other metabolic changes associated with these diets affect tumor growth.
Research contents
Calorie restriction, but not a ketogenic diet, will disrupt the growth of PDAC allograft tumors
The researchers obtained AL1376 pancreatic ductal adenocarcinoma (PDAC) cells from the LSL-Kras(G12D); Trp53fl/fl; Pdx1-Cre mouse model, transplanted them into C57BL/6J mice to form subcutaneous tumors, and exposed the mouse model to controls Under diet, calorie intake, or ketogenic diet. Both diets cause similar reductions in blood sugar levels, and only the ketogenic diet significantly reduces fasting plasma insulin levels. But interestingly, only calorie restriction inhibited tumor growth in this model.
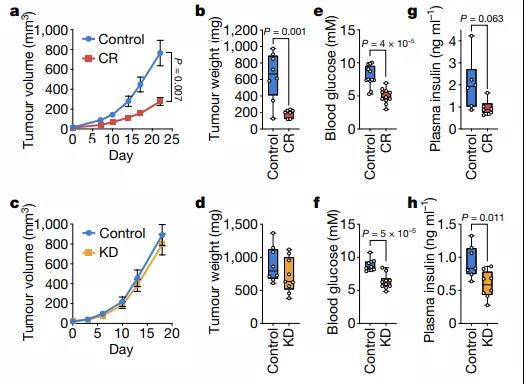
Image source: Nature
Calorie restriction and ketogenic diet differently change the nutrient levels in plasma and tumor interstitial fluid
The researchers tested the levels of metabolites in plasma and tumor interstitial fluid after feeding a control diet, calorie restriction, or ketogenic diet on a mouse model of tumor. It is worth noting that despite lowering blood sugar levels, the ketogenic diet does not reduce the glucose level in the tumor interstitial fluid, while caloric restriction reduces the glucose level in the tumor interstitial fluid from ~1.6 mM to ~0.8 mM.
Why calorie restriction has a stronger effect on glucose levels in tumor interstitial fluid is not yet clear. In this regard, tumor vascularization or tumor cell glucose uptake may have an impact. The effect of calorie restriction on glucose in tumor interstitial fluid may partially help inhibit tumor growth. However, since reducing glucose to 0.8 mM has little effect on cell proliferation, the researchers considered whether changes in the availability of other nutrients might also contribute to the effect of calorie restriction. Calorie restriction and ketogenic diets have little effect on the levels of many metabolites. Most notably, the ketogenic diet is more effective in increasing the level of “ketones”-β-hydroxybutyrate (β-OHB).
Next, the researchers found significant differences in the effects of calorie restriction and ketogenic diets on fatty acid levels. Although many fatty acids in plasma and tumor interstitial fluid increase with the ketogenic diet, the levels of almost all fatty acids are reduced by caloric restriction.
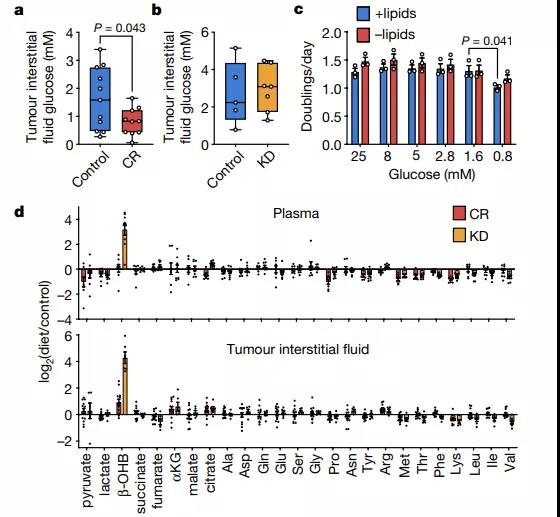
Image source: Nature
Cancer cells need to increase SCD activity to adapt to exogenous lipid restriction
To evaluate how lipid deprivation affects cell fatty acid metabolism, we cultured cells in lipid-depleted media containing only trace fatty acids. Cancer cells derived from various tissues, including AL1376, LGSP, HeLa, Panc1, and A549 cells, can still proliferate in lipid-depleted media, albeit at a slower rate. Lipid depletion will reduce the cellular level of polyunsaturated fatty acids, because mammalian cells cannot synthesize these essential fatty acids and must be obtained from the environment. In contrast, the levels of saturated fatty acids and monounsaturated fatty acids are relatively maintained, consistent with the exogenous lipid restriction that promotes SREBP1 transcription factor activation and fatty acid de novo synthesis.
Interestingly, in cells cultured under lipid-depleted conditions, 16:1 (n-7) MUFA levels increased significantly. Stearoyl-CoA desaturase (SCD) is responsible for the synthesis of 16:1 (n-7) and 18:1 (n-9) monounsaturated fatty acids from 16:0 and 18:0 SFA, respectively. Researchers found that 16: 1 (n-7)/16:0 and 18:1 (n-9)/18:0 ratios are alternatives to SCD activity, which are significantly increased in lipid-starved cells and are inhibited by SCD Agent A939572 is reduced.
Consistently, the flux of lipid-starved cells through SCD was increased. By inhibiting SCD under lipid-depleted culture conditions, cell proliferation was disrupted and the death of AL1376, LGSP, and HeLa cells was induced. Therefore, when the exogenous lipids are low, these cancer cells need to up-regulate SCD activity.
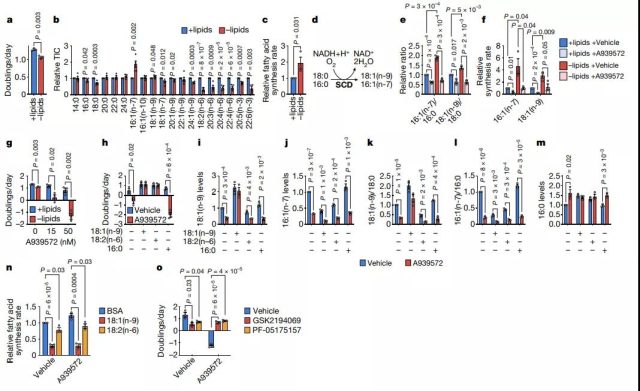
Image source: Nature
A low-glycemic diet can damage tumor SCD, and SCD interacts with changes in lipid availability to affect tumor growth
SCD activity is essential for cancer cell growth in a lipid-depleted environment. When SCD is inhibited, the balance between unsaturated fatty acids and saturated fatty acids is disrupted, and cell proliferation is impaired; when saturated fatty acids accumulate, cell death is induced. Since caloric restriction cannot deplete lipids in the tumor microenvironment to the same extent as lipid-depleted media, these in vitro findings may not fully explain the researchers’ dietary observations.
But despite this, because tumor SCD activity may be destroyed by calorie restriction, it shows how a low-glycemic diet can affect tumor growth. Because AL1376 and LGSP caloric restriction tumors maintain 16:0 and 18:0 levels, but 16:1 (n-7), 18:1 (n-9), 16:1 (n-7)/ 16:0 and 18 :1(n-9)/18:0 The level is reduced.
Consistently, tumors under most calorie-restricted diets have less SCD protein. The tumors of mice on the ketogenic diet also had lower levels of 16:1 (n-7) and 16:1 (n-7)/16:0, and most tumor analyses of the ketogenic diet showed reduced SCD protein levels, indicating The ketogenic diet can also impair tumor SCD activity.
However, unlike caloric restriction, tumors on a ketogenic diet maintain 18:1 (n-9) and 18:1 (n-9)/18:0 levels, possibly because the source of fat in the ketogenic diet is rich in 18 :1 (n-9) lard, which leads to an increase of 18:1 (n-9) levels in plasma and tumor interstitial fluid.
Because SCD inhibition induces cell death only when saturated fatty acids accumulate, these data are consistent with caloric restriction impairing proliferation to slow tumor growth, rather than leading to cell death and tumor regression, and suggest that palmitate toxicity is associated with extreme SCD inhibition.
Not caused by calorie restriction. In contrast, the ketogenic diet improves the availability of tumor lipids. It maintains tumor levels of 18:1 (n-9) and 18:1 (n-9)/18:0, although SCD activity is reduced, but The tumor is still allowed to grow. In summary, these data indicate that the ability of a low-glycemic diet to cause an imbalance between unsaturated fatty acids and saturated fatty acids is the reason that diet affects tumor growth.
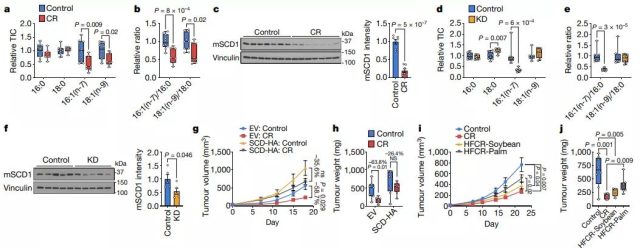 Image source: Nature
Image source: Nature
Summarize
This paper studied the effects of calorie restriction and ketogenic diet in a mouse model of pancreatic cancer, and found that only calorie restriction can inhibit tumor growth.
Calorie restriction, but not a ketogenic diet, lowers lipid levels in plasma and tumors, and reduces the activity of enzymes required for cancer to adapt to a low-lipid environment, which leads to an imbalance between unsaturated and saturated fat.
Although the ketogenic diet also impairs the activity of this enzyme, this diet increases the availability of lipids, thereby maintaining an unsaturated/saturated fat ratio that is conducive to tumor growth.
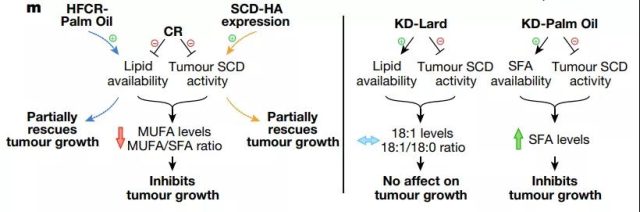 Image source: Nature
Image source: Nature
In addition to the mouse experiments, the authors also evaluated the association between dietary patterns and survival time in 1,165 pancreatic cancer patients. A high-fat and low-carbohydrate diet is associated with longer survival time. However, the author points out that a low-sugar diet is not suitable for all cancer patients. Because they may be difficult to maintain and tolerate, weight loss may affect treatment options.
“There is a lot of evidence that diet affects the rate of cancer progression, but it does not cure,” said study author Matthew G. “While these findings are instructive, they do not indicate that cancer patients should try these diets.” Instead, they believe that these findings are worthy of further research to determine how to combine dietary interventions with existing or emerging drugs to further help cancer patients formulate correct dietary interventions.
Ketogenic diet vs. Calorie restriction diet: Which can limit tumor growth?
References:
https://doi.org/10.1038/s41586-021-04049-2
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



