FDA lifts partial clinical suspension of potent and selective menin inhibitor KO-539!
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
FDA lifts partial clinical suspension of potent and selective menin inhibitor KO-539!
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
FDA lifts partial clinical suspension of potent and selective menin inhibitor KO-539!
Precision therapy for leukemia (AML)! US FDA lifts partial clinical suspension of potent and selective menin inhibitor KO-539!
Recently, Kura Oncology announced that the U.S. Food and Drug Administration (FDA) has lifted a partial clinical hold on the KOMET-001 Phase 1b trial of KO-539 in relapsed or refractory acute myeloid leukemia (AML).
Previously, after Kura reported a grade 5 serious adverse event (patient death) that may be related to differentiation syndrome (DS) to the United States, the FDA suspended the above-mentioned Phase 1b clinical trial in November 2021. The partial clinical hold was lifted following an agreement with the FDA on the company’s DS mitigation strategy.
DS is a known targeting effect associated with differentiation-inducing therapeutics (differentiation-inducing agents) in the treatment of AML. DS is characterized by unexplained fever, acute kidney injury, hypotension, weight gain, and other deleterious outcomes. This adverse event occurs in up to a quarter of patients treated with this class of drugs.
Previously, a report published by foreign biopharmaceutical website FiercePharma pointed out that DS side effects had been included in the black box warning of Agios Pharmaceuticals’ marketed AML drug Tibsovo, which was approved in July 2018. In addition, Astellas AML drug Xospata also has a 1% incidence of DS.
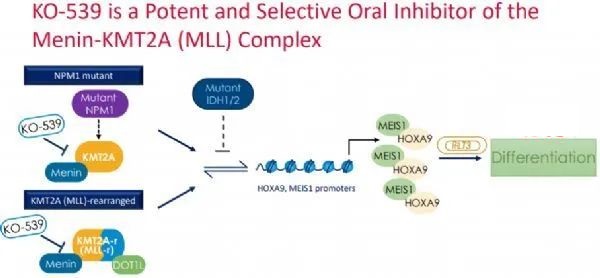
The mechanism of action of KO-539 (Image source: kuraoncology.com)
According to Kura’s website, KO-539 is an oral, potent, selective menin inhibitor developed for the treatment of patients with genetically defined AML, including those with NPM1 mutations or KMT2A rearrangements.
KO-539 blocks the interaction of menin with KMT2A/MLL, two proteins that together are critical for the survival, growth and proliferation of certain leukemia cells.
Available preclinical data support the potential antitumor activity of KOS-539 in genetically defined subsets of acute leukemia, including those with rearrangements or partial tandem repeats in the KMT2A gene, and those with oncogenic driver mutations in genes such as NPM1 group.
These preclinical data support the hypothesis that KO-539 targets epigenetic dysregulation and removes certain barriers to cell differentiation, thereby driving antitumor activity.
It is estimated that KO-539 has the potential to address approximately 35% of AML, including NPM1-mutant AML and KMT2A-rearranged AML.
KOMET-001 (Kura Oncology Menin Inhibitor Trial) is a Phase 1/2 first-in-human open-label clinical trial to determine the safety, tolerability and resistance of KO-539 in patients with refractory or relapsed AML.
KO-539 demonstrated a broad therapeutic window in the phase 1a dose-escalation portion of KOMET-001, with favorable monotherapy activity in all patients with relapsed or refractory AML, including those with NPM1 mutations and KMT2A rearrangements.
The Phase 1b portion includes 2 expansion cohorts – a low dose of 200 mg and a high dose of 600 mg.
Each cohort will enroll 12 patients with NPM1-mutated or KMT2A-rearranged relapsed or refractory AML to evaluate the safety, tolerability, pharmacokinetics, and efficacy of KO-539 to determine the recommended Phase 2 of KO-539 dose.
Reference:
Kura Oncology Receives FDA Authorization to Proceed with Phase 1b Study of KO-539 in Acute Myeloid Leukemia
FDA lifts partial clinical suspension of potent and selective menin inhibitor KO-539!
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



