BioNTech Released Clinical Data of CAR-T+mRNA Vaccine for Solid Tumors
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
BioNTech Released Clinical Data of CAR-T+mRNA Vaccine for Solid Tumors
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
BioNTech Released Clinical Data of CAR-T+mRNA Vaccine for Solid Tumors.
On April 10, 2022, BioNTech announced Phase 1/2 of its CAR-T + CARVac combination in Claudin6 (CLDN6) -positive solid tumors (testicular cancer, ovarian cancer, etc.) at the American Association for Cancer Research (AACR) 2022 Annual Meeting Preliminary clinical data show good safety and encouraging preliminary therapeutic effects .
It is reported that this is also the first human trial to improve the expansion and persistence of CAR-T cells through mRNA vaccines.
CAR-T cell therapy has revolutionized treatment options for hematological malignancies, but its application in solid tumors has been challenging.
One of the major limitations is that most therapeutic targets on solid tumors are also expressed in small amounts in normal cells, making it difficult to specifically target CAR-T cells to tumor cells without harming normal cells.
In addition, the suppressive tumor microenvironment of solid tumors makes it difficult for CAR-T cells to persist and penetrate solid tumors.
This open-label, multicenter human clinical trial is designed to evaluate the safety and preliminary efficacy of CLDN6-CAR-T cell therapy (BNT211) .
CLDN6 is a tumor-specific antigen that is widely expressed in a variety of solid tumors but silenced in adult normal tissues.
BNT211 consists of two parts, an autologous CAR-T cell therapy targeting CLDN6 , and an mRNA vaccine (CARVac) encoding CLDN6 , which is based on BioNTech’s mRNA-lipoplex technology and shares the same technology platform as its COVID-19 mRNA vaccine.
In January 2020, a preclinical study published by BioNTech in Science showed that the combination of CLDN-CAR-T with a vaccine encoding CLDN6 mRNA can promote the expansion of CAR-T cells and increase their persistence, thereby improving tumor susceptibility.
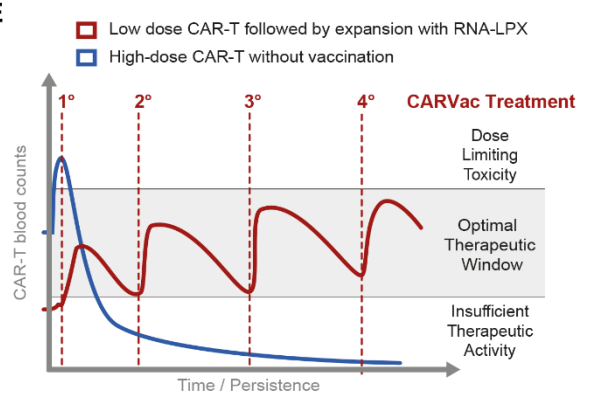
In this clinical trial, the research team recruited 16 patients with relapsed or refractory advanced CLDN6-positive solid tumors to test the safety and preliminary efficacy of CLDN6-CAR-T cell therapy alone, as well as in combination with CARVac.
The 16 patients were testicular cancer (n=8) , ovarian cancer (n=4) , endometrial cancer , fallopian tube cancer , sarcoma , and gastric cancer (1 case each) .
Six weeks after the infusion , of the 14 patients evaluable for efficacy, 4 patients with testicular cancer and 2 patients with ovarian cancer had a partial response (PR) , an objective response rate of nearly 43% .
Of the patients who achieved partial responses, four received CAR-T cell therapy alone and two received the CAR-T-CARVac combination, resulting in a disease control rate of 86% .
At 12 weeks post-infusion, 4 out of 6 patients with previous partial responses had sustained effects, and at week 18, 1 patient with testicular cancer achieved a complete response (CR) .
Controlled cytokine release syndrome occurred in approximately 40% of patients, and none of the patients showed any signs of neurotoxicity.
Other adverse events included cytopenias and abnormal immune responses, all of which resolved.
Administration of CARVac resulted in flu-like symptoms, but only for 24 hours, suggesting that CLDN6-CAR-T therapy and combination therapy with CARVac are safe with limited and manageable adverse events.
These preliminary data show that CLDN6-CAR-T cell therapy alone and CLDN-CAR-T + CAR-Vac combination therapy are safe and show promising therapeutic effects in patients with CLDN6-positive cancers.
Prior to this, CLDN6 has not been used as a cell therapy target, and this clinical trial shows that CLDN6-targeted cell therapy has better therapeutic effect on solid tumors.
It should be pointed out that this clinical trial involves fewer patients and requires larger-scale and longer-term observations in order to draw more accurate conclusions.
References :
https://investors.biontech.de/news-releases/news-release-details/biontech-presents-positive-preliminary-phase-12-data-first-class
BioNTech Released Clinical Data of CAR-T+mRNA Vaccine for Solid Tumors
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



