What is the relationship between Harmful metabolites of TME and antitumor immunity?
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
What is the relationship between Harmful metabolites of TME and antitumor immunity?
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
What is the relationship between Harmful metabolites of TME and antitumor immunity?
Cancer immunotherapy has revolutionized the way cancer is treated, opening the door to durable disease-free states for a subset of patients.
This major advance comes with a greater understanding of how the immune system interacts with cancer and the major barriers to successful antitumor immunity.
One such obstacle is the harsh metabolic environment of the tumor microenvironment ( TME ).
It is well known that metabolic disturbances in tumor cells lead to hypoxic, acidic, glucose, and amino acid-deficient environments. Many tumors undergo “Warburg metabolism,” or aerobic glycolysis.
In addition, rapid proliferation and abnormal cell signaling lead to inadequate vasculature, resulting in poor TME oxygenation.
Metabolites produced by these processes are important in shaping immune cell function and response to immunotherapy.
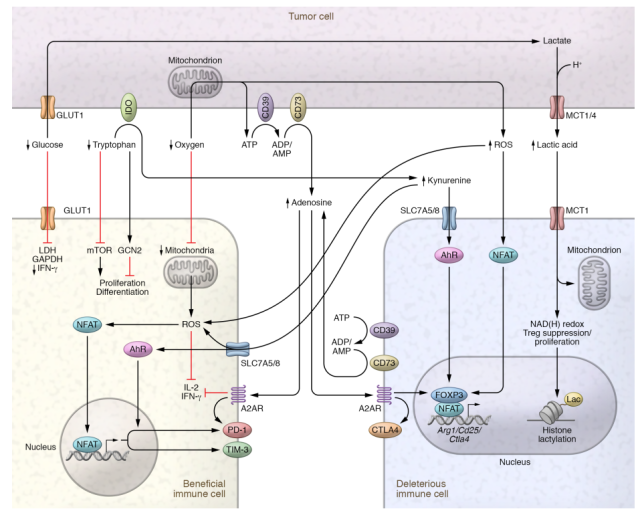
Therefore, we can no longer rely on a simple tumor-starved immune cell model, and must also consider the effects of “toxic” catabolites that are produced in shaping immune cell function.
Catabolites such as lactate, kynurenine, adenosine, and reactive oxygen species ( ROS ) are frequently present in a variety of tissues and immune environments, and it is these non-tumor environments that shape immune cells evolutionarily.
Therefore, it is important to consider the TME as one of the many metabolic environments of immune cells and to seek metabolic insights about tumor-infiltrating lymphocytes from the non-tumor environment.
This perspective is critical for implementing metabolic strategies to improve immunotherapy, as it will shed light on how these treatments may affect the immune system.
Lactic acid
In the TME, lactate is fermented by highly glycolytic tumor cells from glucose to pyruvate, which is then converted to lactate by lactate dehydrogenase ( LDH ).
In normal serum, lactate concentrations ranged from 1.5 to 3 mM, whereas tumor concentrations ranged from 10 to 30 mM, reaching extremely high levels ( 50 mM ) within necrotic tumor cores .
In fact, elevated lactate levels are indicative of poor prognosis in several cancer types. It is therefore not surprising that lactate has profound effects on infiltrating immune cells.
In general, lactate acts as an immunosuppressive metabolite, and indeed, in vitro activation of CD8+ and CD4+ T cells in tumor-equivalent concentrations of lactate reduces their ability to proliferate and produce cytokines.
Lactate restricts T cell proliferation through the NAD(H) redox state, reducing NAD+ to NADH under lactate-rich conditions, thereby altering NAD+-dependent enzymatic reactions, thereby reducing glycolytic intermediates required for proliferation.
Not all immune cells respond negatively to tumor-derived lactate.
The TME actively recruits and promotes the differentiation of Tregs, which are potent suppressors of the immune system, tasked with maintaining immune homeostasis and preventing autoimmunity.
Unlike effector cells, Tregs do not rely on glycolysis to meet metabolic demands, but rely more heavily on oxidative metabolism, including lipid synthesis and signaling.
This allows Tregs to thrive in the glucose-depleted TME and exert their immunosuppressive functions.
Compared with effector T cells, lactate is essential for tumor-infiltrating Treg proliferation and function.
Lactic acid also affects innate immune cells. Lactate was found to polarize macrophages into an M2-type TAM state, including increased arginase 1 ( Arg1 ) expression.
A potential mechanism by which lactate affects macrophage and Treg function may be through its contribution to histone lactylation, thereby altering epigenetics.
The study found that histones can be modified by lactylation, a histone mark with distinct kinetic characteristics compared to acetylation.
Kynurenine
Another metabolite that is consistently up-regulated in multiple tumor types is kynurenine.
Like lactate, kynurenine is an immunosuppressive byproduct derived from depletion of a key metabolite ( tryptophan ).
The consumption of tryptophan and the production of kynurenine are driven by three rate-limiting enzymes, indoleamine 2,3-dioxygenase 1 ( IDO1 ), IDO2 and tryptophan 2,3-dioxygenase ( TDO ).
IDO1 is expressed by a variety of cell types, including immune cells, epithelial cells, cancer cells, and fibroblasts.
IFN-γ produced during tissue inflammation greatly enhances IDO1 expression and acts as a negative feedback loop to suppress excessive inflammation.
Like glucose and lactate, tryptophan depletion and kynurenine production have independent immunosuppressive effects. Kynurenine can be inhibited by a variety of mechanisms, including (a) promoting differentiation of tolerant antigen-presenting cells; (b) promoting Treg differentiation through the aryl hydrocarbon receptor (AhR); and (c) inhibiting IL-2 signaling .
It has been demonstrated that many tumor types express IDO1, and high expression is associated with poor prognosis and increased tumor-infiltrating Tregs.
Kynurenine can also directly affect effector T cells, as T cell receptor ( TCR ) stimulation can increase kynurenine uptake via Slc7a5/Slc7a8, resulting in AhR-induced increase in PD-1 expression.
Taken together, tryptophan depletion and kynurenine production are used to create an immunosuppressive environment that maintains immune tolerance at steady state but is used in tumors to evade immune destruction.
Reactive oxygen species
Many tumors experience some degree of hypoxia. Oxygen depletion occurs when the tumor’s low vascularization and high metabolic demands exceed the available oxygen supply.
Like the consumption of glucose and tryptophan, the consumption of oxygen is accompanied by the production of toxic by-products, such as reactive oxygen species and adenosine, which have long been the focus of cancer research.
ROS are produced as a normal part of oxidative metabolism and are important for normal cell survival, signaling, and homeostasis.
Cancers, however, utilize reactive oxygen species and use their overproduction to drive mitotic signaling, metastasis, and survival.
In addition to reactive oxygen species, tumor hypoxia promotes the accumulation of extracellular ATP, which is broken down into the immunosuppressive metabolite adenosine.
ATP release and adenosine production act on purinergic receptors, impairing immune cell infiltration and activation, thereby reducing antitumor immunity.
Like lactate and kynurenine, reactive oxygen species play an important role in shaping immune cell function in a non-tumor environment.
Reactive oxygen species can also play a role in chemotaxis, signaling neutrophils and other immune cells to reach sites of injury or infection, and even activating dendritic cells.
However, high levels of ROS can damage effector T cells within the TME.
A more oxidized TME was associated with increased CD8+ T cell depletion and decreased response to anti-PD-1 immunotherapy.
Recent mechanistic studies have shown that sustained TCR stimulation and hypoxia prompt CD8+ T cells to produce more mitochondrial ROS, which is sufficient to induce an exhaustion-like phenotype.
In addition to damaging effector cells, high levels of ROS can support regulatory populations such as TAMs and MDSCs.
In conclusion, reactive oxygen species are essential for normal immune function at low levels, but at high levels of the TME, reactive oxygen species promote the dysfunction of effector cells and the presence of regulatory populations.
Adenosine
Adenosine is a potent immunosuppressive metabolite that both damages effector cells and supports regulatory cells.
Within the TME, adenosine is produced by the extracellular nucleotidase CD39 and CD73 expressed by tumor cells and infiltrating immune cells.
Hypoxia drives HIF1A activity, which in turn upregulates CD39, CD73, and the adenosine receptor A2BR.
Extracellular ATP is converted to ADP and/or AMP by CD39, while AMP is converted to adenosine by CD73.
Adenosine then binds to one of the four receptors, A1R, A2AR, A2BR or A3R, to exert its regulatory function.
Signaling through A2AR and A2BR reduces IFN-γ and IL-2 production, increases the inhibitory molecule PD-1 in effector cells, and activates Foxp3, CTLA4, and Lag-3 to promote Treg development.
Targeted therapy against toxic metabolites
The production and immunomodulatory effects of lactate, kynurenine, reactive oxygen species, and adenosine in tumors are recognized, and treatments for them have been developed with a view to improving cancer immunotherapy.
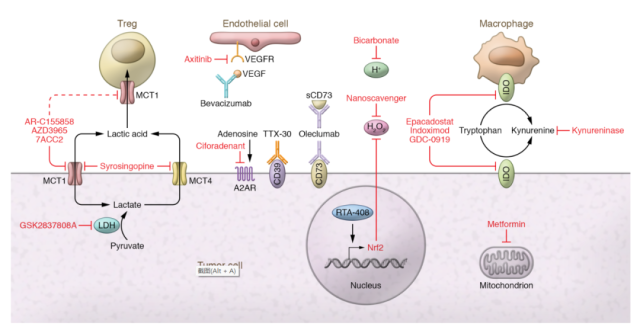
Alter tumor metabolism
Numerous strategies exist to alter the metabolic profile of tumors. For example, lactate production can be achieved by inhibiting lactate dehydrogenase.
A drug study showed that inhibition of patient-derived and B16 melanoma LDHA with the inhibitor GSK2837808A enhanced T cell killing in vitro and in vivo and enhanced adoptive cell therapy.
Although specific LDH and glycolysis inhibitors have not yet fully entered the clinic, it is possible to repurpose older drugs with glycolysis inhibition.
For example, diclofenac , a common NSAID, has been shown to modulate glycolysis independent of COX inhibition and can be used to improve anti-PD-1 immunotherapy.
Lactic acid can also reduce lactate within the TME by targeting its export. Lactate is transported through MCTs, and while many small-molecule inhibitors of MCT1 and MCT4 have been developed for preclinical use, only AstraZeneca’s AZD3965 is currently in human trials ( NCT01791595 ).
Preclinical studies have shown that AZD3965 can reduce lactate secretion into the TME and increase tumor immune cell infiltration.
Most of the research on kynurenine has been done by inhibiting IDO1. Currently, several IDO inhibitors are in clinical trials ( NCT04049669, NCT03432676, NCT02471846 ).
Unfortunately , Incyte’s trial of epacadostat in combination with pembrolizumab was stopped after an interim analysis showed no additional benefit from IDO1 inhibition ( NCT03432676 ) .
This certainly dampened enthusiasm for targeting IDO1, but it highlights the complexity of targeting the IDO pathway, presumably requiring a certain level of tryptophan catabolism for optimally regulated antitumor immune responses.
Due to the diversity of ROS production mechanisms, ROS production can be targeted in a variety of ways. One promising approach is by reducing tumor hypoxia.
In preclinical models, metformin acts as a weak mitochondrial complex I inhibitor that, when combined with anti-PD-1, reduces tumor hypoxia and promotes B16 tumor clearance.
Tumor hypoxia can also be targeted by inhibiting VEGF, and clinically, an anti-angiogenic and immunotherapy combination has been most effective in renal cell carcinoma ( RCC ) and hepatocellular carcinoma ( HCC ), with a combination of atezolizumab and bevacizumab Progression-free survival and overall survival are improved.
Another way to target tumor-derived reactive oxygen species is to use scavengers. An example is the use of the drug RTA-408 , which was shown to induce Nrf2, a major protein involved in oxidative stress protection, and lead to inhibition of ROS in tumor xenograft models.
In 2019, a Phase Ib/II clinical trial combining anti-CTLA-4 and anti-PD-1 with RTA-408 was completed in melanoma patients, but the results have not been officially published ( NCT02259231 ).
In addition to the aforementioned methods of reducing hypoxia, it is also possible to directly target adenosine production.
These drugs come in the form of small molecule inhibitors or blocking antibodies, mainly targeting CD73, CD39 and A2AR.
While these drugs have shown preclinical efficacy in reducing adenosine production and even preventing soluble CD73 exonucleotidase, most are still awaiting results from phase I/II clinical trials.
Altered metabolism of infiltrating immune cells
Adoptive T-cell therapy such as CAR-T offers a way to metabolically support T-cell function in the harsh TME. One way to boost CAR-T cells is to overexpress or delete genes that regulate metabolism.
For example, in adoptive cell therapy models, overexpression of PGC1α, a transcriptional coactivator of mitochondrial biogenesis, prevents loss of mitochondrial function and improves antitumor efficacy.
Conversely, deletion of the Regnase-1 gene increased the efficiency of adoptive cell transfer. The Regnase-1 gene is thought to negatively regulate the transcription factor BATF and mitochondrial metabolism in CD8+ T cells.
Adoptive T-cell therapy can also provide metabolic support through its expansion mediators.
Compared with serum, commonly used media such as RPMI, DMEM, and AIM V contain a large amount of glucose, reduce the level of metabolites, and are insufficiently prepared for the harsh metabolic environment of the tumor.
Because T cells are highly sensitive to their metabolic environment, expansion of T cells in media containing or lacking certain metabolites may improve their persistence and potency in vivo.
Consistent with this, in vitro glutamine restriction by nutrient starvation or metabolic inhibitors enhanced the efficacy of adoptively transferred T cells in mice.
Restricting metabolites or adding metabolic inhibitors to expansion media is a very attractive approach to improve adoptive cell therapy.
Summary: What is the relationship between Harmful metabolites of TME and antitumor immunity?
Tumors not only consume essential metabolites, but also produce toxic byproducts that persist in the TME.
Depletion of metabolites such as glucose, amino acids, and oxygen , as well as production of lactate, kynurenine, reactive oxygen species, and adenosine, negatively regulate effector immune cells and support regulatory immune populations.
As we further investigate immune metabolism in the TME, it is critical to understand that both consumption and production of metabolites have independent effects on immune cell function.
As the IDO1 inhibitor trial failed, there may be a more delicate balance than we thought between the depletion of essential metabolites and the production of toxic metabolites.
Ultimately, understanding the physiological balance between essential metabolites and their toxic byproducts, and subsequent effects on immune cells, will be key to developing cancer treatments.
references:
1. Fighting in a wasteland: deleterious metabolites and antitumor immunity. J Clin Invest. 2022 Jan 18; 132(2):e148549.
What is the relationship between Harmful metabolites of TME and antitumor immunity?
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



