A scientist who has studied Parkinson’s for 30 years develops Parkinson’s disease
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
A scientist who has studied Parkinson’s for 30 years develops Parkinson’s disease
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
A scientist who has studied Parkinson’s for 30 years develops Parkinson’s disease, he says there is hope.
A few years ago, Dr. Tim Greenamyre, director of the Institute for Neurodegenerative Diseases at the University of Pittsburgh, began noticing some worrisome symptoms in himself— he couldn’t smell anything, and he had Constipated condition;
moreover, he started yelling and kicking in his sleep, unable to swing his left arm when walking.
Tim is also a neuroscientist and physician, and these symptoms are not unfamiliar to him-do you have Parkinson’s disease (PD) ?
In July 2021, Tim turned to a neurology colleague to confirm this concern. The reality is harsh – he does suffer from Parkinson’s disease .
In the past 30 years, he has been committed to researching the treatment and even the cure of Parkinson’s disease (PD) .
During his long and fruitful career, the 67-year-old has not only earned the respect of his patients and clinical colleagues, but has also developed a widely used animal model of PD and has been instrumental in identifying the environmental triggers of PD. made a key contribution.
However, this research work exposed him to chemicals that induce PD in rodents, which is likely a contributing factor to his own disease.
In response, Tim said helplessly: “It’s very ironic.” To those unfamiliar, he showed almost no signs of illness. So far, Tim said medication has helped.
Adding to the irony is that Tim was diagnosed with PD at a time when researchers who specialize in PD see new promise in the field .
Still, researchers think they may finally be able to find a way to slow or block the second most common neurodegenerative disease after Alzheimer’s disease .
The past and present of Parkinson’s disease
In the United States, about 1 million people are troubled by PD, and nearly 90,000 new PD cases are added every year. Globally, more than 8.5 million people suffer from PD, and statistics show that it is the fastest growing neurological disorder in the world.
The typical symptoms of PD were first described and recorded by British surgeon James Parkinson in 1817, so it was named “Parkinson’s disease”. PD is caused by the degeneration of cells that produce the neurotransmitter dopamine in the substantia nigra of the brain.
Since the substantia nigra is a region of the midbrain involved in motor control, its lesions can lead to a variety of movement-related symptoms, including the most common tremors, muscle stiffness, As well as issues of balance and coordination .
As the disease progresses, people may have trouble speaking and voluntary movement. Afterwards, many people develop dementia. PD itself is not fatal, but its complications—particularly aspiration pneumonia due to difficulty swallowing—often kill.
Dopamine, usually administered as an oral drug combination of levodopa (L-Dopa) and carbidopa, has been the first-line therapy for PD since it was approved in the United States in the 1970s. Dopamine-based drugs can improve motor symptoms in PD patients, but over time, their effects begin to wear off rapidly, and there are sometimes excruciating side effects, including dyskinesias—that is, involuntary, twitching movements.
Fifty years after the introduction of dopamine drugs, scientists have still not been able to find a treatment that stops the disease from progressing and not just its symptoms, leaving patients and their families miserable and frustrated.
“There’s never been a better time to be diagnosed with Parkinson’s disease”
Now, scientists believe that “we are at an inflection point”, this optimism stems from the accumulation of decades of research.
Since 1997, gene mutations related to PD have been discovered one by one, opening the door for scientists to explore the molecular mechanism of PD, and providing a theoretical basis for pharmaceutical companies to develop PD drugs and other experimental therapies. Now, these therapies are entering clinical trials at an astonishing pace.
“There has been a shift in the field of PD as we better understand the disease and its targets,” the researchers said. More than 50 clinical trials targeting the root causes of PD are now underway, compared with 15 years ago Clinical trials are few and far between, which is a remarkable advance.
Despite these promising signs, the expected breakthrough may come too late for PD patients like Tim. PD may have progressed quietly over decades when people notice trembling hands or limited mobility, leading to milder symptoms such as constipation and loss of smell, and destroying vast numbers of dopamine-producing neurons in the substantia nigra .
Although the claws of PD have reached out to him, Tim is still determined to defeat it. ” Being diagnosed with Parkinson’s disease is never good news, but if you had to pick an era, now is the best time in history to be diagnosed with Parkinson’s disease,” he said when he received the Fox Foundation’s Research Leadership Award last fall . “
Commonly used pesticides are the “culprit” behind non-genetic Parkinson’s disease
Tim first came into contact with PD research when he was a student at the University of Michigan School of Medicine in his early years.
At that time, a young assistant professor described his work during his Ph.D.——clarifying how strychnine (strychnine) exerts its effect on the neurotransmitter glycine receptors in the spinal cord.
This work made Tim Became fascinated by research in the field of neuroscience. “I thought it was so cool to explore basic pharmacological and physiological mechanisms with clinical implications,” he recalls.
To this end, Tim joined the professor’s lab and developed a technique to visualize neurotransmitter receptors in the brain for studying the mechanisms of Alzheimer’s and Huntington’s diseases.
Using this as a starting point, Tim obtained a Ph.D., established his own laboratory, and entered a top movement disorder clinic at the University of Rochester to treat patients, starting his career in the fight against Parkinson’s disease.
Like many academics who have stepped into the field of PD research, Tim was inspired by a 1983 Science paper that described a group of young patients in a Northern California hospital who had a sudden onset of PD, all taking a drug . A drug called MPTP, whose toxic metabolite MPP+ destroys dopaminergic neurons in the substantia nigra.
Following this lead, the scientists soon discovered that MPP+ inhibits complex I (complex I) , a key enzyme in the metabolic process involved in the energy conversion of mitochondria . This finding suggests that mitochondrial damage may play a role in PD pathology.
MPP+ is not the only inhibitor of complex I, there are many other chemicals that may have this effect. But what caught the scientists’ attention was that the classic inhibitor of this important mitochondrial enzyme is a pesticide called rotenone, which is commonly used in people’s gardens or vegetable growing .
Rotenone is considered a “green organic” insecticide because it is a natural insecticide extracted from the roots of certain plants. People also use it to kill fleas and ticks on pets, and the Department of Wildlife uses it to control invasive fish populations.
For Tim, it was a gateway to the mysteries of PD. By 1990, he was using radiolabeled rotenone to determine the localization of complex I in the brain, a molecule that readily crosses biological membranes (including the blood-brain barrier) due to its lipophilic nature .
Around the same time, some laboratories found evidence in brain autopsy samples, as well as in platelets from PD patients, that there was a defect in complex I activity in the mitochondria of PD patients .
This led Tim to realize that he could mimic PD by exposing rats to rotenone. He expected rotenone to increase the number of mitochondria in every organ—and it did—but it only had a massive toxic effect on one cell type, the dopamine-producing neurons in the substantia nigra.
In 2000, Tim and his team published a seminal paper in Nature Neuroscience.
They report that chronic intravenous administration of rotenone to rats selectively destroys dopamine-producing neurons in the substantia nigra that also degenerate in PD patients .
In addition, surviving neurons contained fibers, or clumps of alpha-synuclein aggregates. These aggregates closely resemble the structure of Lewy bodies, one of the hallmarks of PD in the human brain.
What’s more, these rats developed symptoms of PD after the rotenone injection, such as unsteady movements and hunching, paw shaking and severe rigidity .
 A scientist who has studied Parkinson’s for 30 years develops Parkinson’s disease
A scientist who has studied Parkinson’s for 30 years develops Parkinson’s disease
This work provides researchers with the first animal model that mimics the classic motor symptoms of PD while exhibiting hallmark pathological features. It also fueled suspicions that rotenone and other pesticides could trigger PD .
Tim now wonders whether his decades of research on rotenone and similar compounds may have contributed to his PD.
Rotenone must be dissolved in a solvent such as dimethyl sulfoxide (DMSO) to make an infusible solution, he explained .
The solution would spill on his gloves from time to time, and the DMSO allowed it to pass right through the gloves and into the skin .
At the time Tim and his colleagues didn’t see it as dangerous, and a high-quality epidemiological study published in 2011 showed that rotenone increased the risk of PD in farmers and their spouses by a factor of 2.5.
What’s wrong with redheads? Uncovering the genetic basis of Parkinson’s disease
If rotenone did play a role in causing Tim’s disease, it likely increased an underlying genetic predisposition.
Tim is one of the 90 percent of patients with “idiopathic PD” — patients who have no clear genetic cause but who are certain that their disease is caused by some unclear combination of genetic susceptibility and environmental triggers.
For example, Tim used to have red hair, which epidemiological studies have linked to an increased risk of PD, though the reasons behind this association are unclear.
About 10 percent of PD cases are due to mutations in specific genes. In 1997, an article published in the journal Science identified the first causative gene for PD—the gene encoding synuclein. Since then, many scientists have focused their attention on the genetic research of PD. Soon, they found more mutations in PD-associated genes, including the gene encoding Parkin protein, the gene encoding LRRK2 enzyme, and the gene encoding glucocerebrosidase. Scientists are beginning to delve deeper into how these genetic mutations cause nerve damage.

A scientist who has studied Parkinson’s for 30 years develops Parkinson’s disease
When similar genetic discoveries flooded in, Tim was struggling with a changing family life and an underfunded lab.
Despite the difficult circumstances, Tim has been trying to find the key mechanism of destroying neurons in the pathogenesis of PD, rather than following the trend and chasing new disease-causing genes.
He has been focusing on the interactions between genes and the environment, especially those pesticides and solvents that interact with genes associated with PD.
In recent years, Tim has focused on the gene LRRK2 , which encodes an enzyme that is a master “traffic controller” that regulates the movement of proteins and vesicles within cells.
Mutations in this gene lead to abnormally elevated activity of this enzyme, which ultimately impairs the function of lysosomes—the “garbage dump” cells use to degrade unwanted proteins. Damage to lysosomes is thought to be a sign of PD. one of the causes .
LRRK2 mutations account for approximately 3% to 4% of all PD cases.
In 2021, Tim’s research group found that the toxin can mimic the adverse effects of LRRK2 mutations. They demonstrated that the solvent trichlorethylene , a mitochondrial toxin used in dry cleaning and metal degreasing, increased the activity of the LRRK2 enzyme and induced PD-like pathology in the brains of aged rats .
The work provides fodder for an initial class-action lawsuit against groups who drank from contaminated water decades ago at the Marine Corps base at Camp Lejeune, North Carolina. They claimed that the trichlorethylene in the water caused their PD.
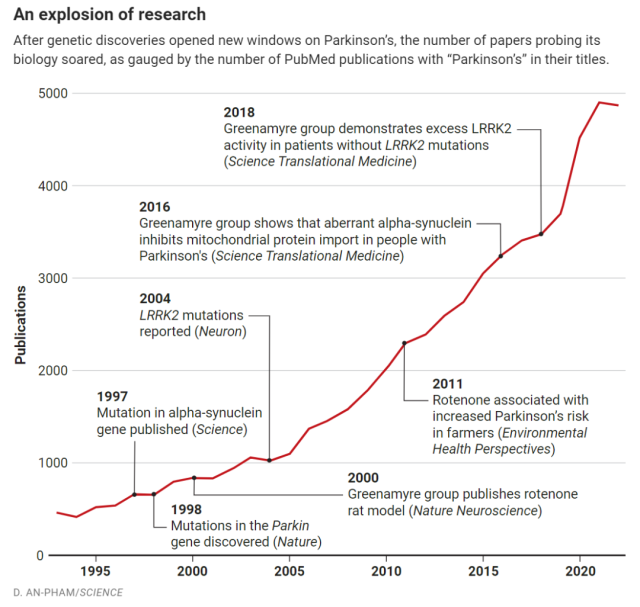 Representative research progress in the field of Parkinson’s disease
Representative research progress in the field of Parkinson’s disease
Tim’s decades-long work on environmental toxins provides the basis for a lawsuit brought against the EPA by the Fox Foundation and others after the U.S. Environmental Protection Agency (EPA) reapproves widespread use of paraquat in 2021 .
Like rotenone, paraquat can cause PD symptoms and pathological changes in rodents, and other researchers have also used it to make PD animal models.
The EPA argues that, with safety measures in place, the agricultural benefits of paraquat outweigh its risks to human health and maintains that there is “insufficient epidemiological evidence” of a causal link to PD.
Tim filed an amicus brief in the case, accusing the EPA of promoting paraquat (which has been banned in at least 50 countries) without considering the results of epidemiological and animal studies .
Tim’s study also prompted the EPA to review the safety of rotenone again. In 2007, the EPA restricted the use of rotenone to control invasive fish species, and manufacturers voluntarily withdrew household rotenone products from the market.
Last year, the EPA imposed further safety restrictions on its use in fish killing and reiterated that the occupational risks posed by skin exposure to rotenone were “concerning.” But the EPA reviewed the research literature in 2022 and concluded there was “insufficient evidence” of a causal link between rotenone and PD.
Other scientists disagree with this conclusion, arguing that the results of epidemiological studies and the work of scholars such as Tim are sufficient to show that rotenone and other pesticides are important triggers of idiopathic PD .
How long will it take for a new treatment for Parkinson’s disease?
In a clinic in the building next door to the lab, Tim put on a white coat and started clinical outpatient work. Like nearly 1 million PD patients worldwide, Tim’s patients are waiting for new treatments other than dopamine. As one of the patients said: This is still a disease for which there is no cure. Every time I see a doctor, I ask, is there any treatment that is close to success?
A new paper from Tim’s team points to one possible approach. They developed a method to measure the activity of the LRRK2 enzyme in different types of brain cells and further found that the enzyme was overactive in dopamine-producing neurons in the substantia nigra, even in patients without mutations in the LRRK2 gene .
In addition, they revealed the underlying molecular cascade signaling that ultimately leads to the paralysis of lysosomal function.
This, the study suggests, leads to the accumulation of abnormal alpha-synuclein . The industry spoke highly of this work and praised ” this is the first data in patient tissue to prove that there is indeed an upregulation of LRRK2 kinase activity in patients with idiopathic PD .”

The paper suggests that inhibiting LRRK2 enzymatic activity may treat many PD patients — not just those with LRRK2 mutations .
The researchers found that when the rats were injected with rotenone, the LRRK2 enzyme inhibitor suppressed all abnormalities caused by rotenone .
The discovery provides new impetus for pharmaceutical companies to test LRRK2 inhibitors in humans. Among them, Denali Therapeutics’ drug candidate DNL201 is progressing most rapidly.
The company has partnered with Biogen and is currently recruiting PD patients , with or without LRRK2 mutations , for a clinical study to see if inhibitors of the enzyme slow PD progression.
The early clinical results of this therapy show that no matter when single or multiple doses of DNL201 are administered, the inhibition of LRRK2 kinase is observed without adverse reactions.
After administration, LRRK2-related pathways in subjects are affected, and the downstream pathways Lysosomal biomarkers were altered. In addition, the drug has blood-brain barrier penetration properties.
In addition to Denali, several research groups and pharmaceutical companies are actively seeking other PD therapies. Some drugs target proven causes of PD, such as increasing glucocerebrosidase activity.
Other drugs have a strategy of repairing the brain, such as for better repair and maintenance of dopaminergic neurons. But these attempts are not satisfactory, often accompanied by failure.
Researchers hope to reduce failures in PD clinical trials. Given that the underlying biology of PD varies widely across patient populations, researchers are working to match patients to clinical trials so that trials can be conducted in those most likely to respond to therapy .
Identifying high-risk groups for PD before the onset of motor symptoms will also be helpful for the clinical treatment of PD . For example, giving patients early treatment with antibodies against alpha-synuclein, before widespread neuronal loss occurs, might stop the disease from progressing.
But this vision is inseparable from reliable biomarkers. For this reason, the Fox Foundation is conducting a large-scale long-term study to find imaging, biological and genetic biomarkers that can help identify patients in the early stages of PD disease.
The study recently achieved a landmark result and was reported in detail in The Lancet Neurology.
The researchers found that a spinal tap test for misfolded alpha-synuclein in spinal fluid could accurately diagnose PD 88 percent of the time, identifying PD patients even before motor symptoms appeared .
Although the test’s invasive nature prevents it from being routinely used, the scientists say it remains an invaluable tool for screening patients for clinical trials and probing the biology of the disease . And, if early results are positive, similar blood tests may become available as treatments for early-stage PD mature.

Hopes in future
After months of worrying about his anosmia and other symptoms, Tim finally turned to a trusted colleague, Dr. Edward Burton, a neuroscientist who studies PD.
Tim’s symptoms started to appear as early as 2019 at the Gordon Research Conference (Gordon Research Conference) , when Tim served as the moderator of the conference . When someone asked a question, Dr. Burton observed Tim turning his head to the questioner The speed is too slow .
Tim has had a sleep disorder for several years – REM sleep behavior disorder (RBD) which is not a good sign, RBD is an early symptom of PD, but it could also be a rarer, more rapidly progressive neurodegenerative disease harbinger of disease. After listening carefully to Tim’s description of his symptoms, Dr. Burton administered an exercise test for him.
There was a slight lag in Tim’s left hand as he tried to quickly open and close his index finger and thumb. Also, his gait is asymmetrical – he doesn’t swing his left arm as much as he swings his right.
Based on the presentation of his symptoms and the slow progression of his disease, Dr. Burton determined that Tim had PD, rather than something more serious. When he was treated with dopamine, Tim’s symptoms improved rapidly—further confirming the diagnosis.
Tim admitted that he was a little apprehensive about what might happen next. “When I have some weird feeling – everyone probably has these feelings, now I wonder if that’s part of the disease…”
Still, he remains optimistic, as patients ask him if better treatments are on the horizon.
Talking about the future prospects of PD therapy, Tim said, I think we are getting close to the heart of the matter. But it will still take some time before it can be put into large-scale application. Hope is at hand .
Reference: A scientist who has studied Parkinson’s for 30 years develops Parkinson’s disease
https://www.science.org/content/article/twist-fate-what-happens-when-top-parkinson-s-researcher-gets-disease
A scientist who has studied Parkinson’s for 30 years develops Parkinson’s disease
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



