Scientists use immortal stem cells to cultivate artificial meat
- WHO Releases Global Influenza Vaccine Market Study in 2024
- HIV Infections Linked to Unlicensed Spa’s Vampire Facial Treatments
- A Single US$2.15-Million Injection to Block 90% of Cancer Cell Formation
- WIV: Prevention of New Disease X and Investigation of the Origin of COVID-19
- Why Botulinum Toxin Reigns as One of the Deadliest Poisons?
- FDA Approves Pfizer’s One-Time Gene Therapy for Hemophilia B: $3.5 Million per Dose
Scientists use immortal stem cells to cultivate artificial meat
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Scientists use immortal stem cells to cultivate artificial meat, which may achieve unlimited supply in the future.
For cellular agriculture — the technique of growing meat in bioreactors — to successfully feed millions of people, technologists must overcome a number of technical hurdles.
Muscle cell production from sources such as chicken, fish, and cattle needs to increase to millions of tons per year.
Researchers at Tufts University Center for Cellular Agriculture (TUCCA) are taking a step toward this goal by developing immortalized bovine muscle stem cells (iBSCs).
These cells have a rapid growth rate and the ability to divide hundreds of times, possibly indefinitely, further increasing the potential for large-scale meat production.
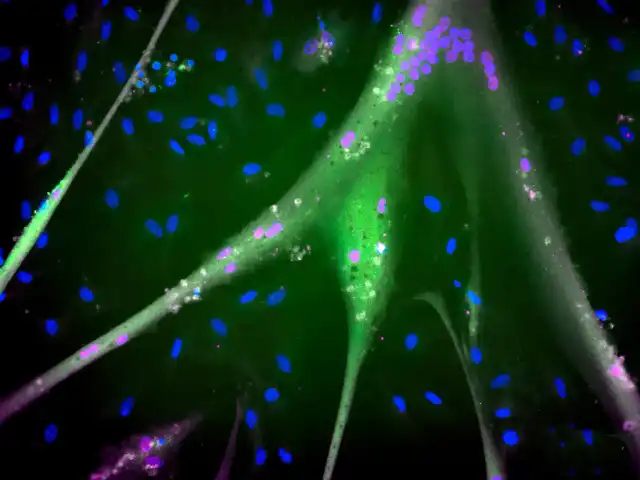
Differentiated immortalized bovine stem cells fully express muscle proteins (blue = nucleus; magenta = troponin; green = myosin). Scale bar about 1 mm. Source: Andrew Stout, Tufts University
The advance, described in the journal ACS Synthetic Biology, means that researchers and companies around the world can obtain and develop new products without having to repeatedly harvest cells from living farm animal tissue.
The production of cell-cultured meat requires muscle and fat cells that are extremely capable of growing and dividing. While cell-grown meat has attracted media attention, such as the US Food and Drug Administration’s initial approval of cultured chicken and even burgers grown with mastodon DNA, these products are still expensive and difficult to scale up.
Normal muscle stem cells taken from living animals typically only divide about 50 times before they begin to “age” and are no longer viable. While these stem cells could theoretically produce large amounts of meat, the immortalized cells developed by the TUCCA team have several advantages. One is that more meat can be produced.
Another advantage is that by making immortalized cells widely available, they will lower the barrier of entry for other researchers exploring cell agriculture—finding ways to reduce costs and overcome the challenges of scaling up production.
“Typically, researchers have to isolate stem cells from animals themselves, which is expensive and laborious, or use stem cells from less related species,” said Andrew Stout, a graduate student at TUCCA and lead researcher on the project. Model cell lines, such as mouse muscle cells.”
There are two key steps in transforming ordinary bovine muscle stem cells into immortal bovine muscle stem cells. As most cells divide and age, DNA at the ends of chromosomes begins to shed, called telomeres. This can lead to errors in DNA replication or repair. It also causes gene loss and eventually cell death.
The bovine stem cells designed by the researchers can continuously rebuild telomeres, effectively keep chromosomes “young”, and prepare for a new round of replication and cell division. The second step in making cells immortal is to make them continue to produce a protein that stimulates a critical phase of cell division. This effectively speeds up the cell division process and helps cells grow faster.
Muscle stem cells are not the end product people want to eat. Not only do they divide and grow, but they also differentiate into mature muscle cells, like, or at least very similar to, the muscle cells we eat in our steaks or fish steaks.
Stott and his research team found that the new stem cells did differentiate into mature muscle cells, although not identical to animal muscle cells or muscle cells derived from traditional bovine stem cells.
“They have the potential to mature enough to replicate the flavor and texture of natural meat,” Stott said. “They are doubling at a very rapid rate, so they may just need a little more time to reach full maturity.”
David Kaplan, the Stern Family Professor of Biomedical Engineering at Tufts University and director of TUCCA, said: “While some may question whether it is safe to ingest immortalized cells, the fact is that when cells are harvested, stored, cooked and digested, they are There’s no viable way to continue growing. Like the natural meat we eat today, the cells just become inert, and we want it to taste good and provide a variety of nutrients.”
Scientists use immortal stem cells to cultivate artificial meat
(sourceinternet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



