Roche’s Faricimab: First ophthalmic bispecific antibody
- Why Lecanemab’s Adoption Faces an Uphill Battle in US?
- Yogurt and High LDL Cholesterol: Can You Still Enjoy It?
- WHO Releases Global Influenza Vaccine Market Study in 2024
- HIV Infections Linked to Unlicensed Spa’s Vampire Facial Treatments
- A Single US$2.15-Million Injection to Block 90% of Cancer Cell Formation
- WIV: Prevention of New Disease X and Investigation of the Origin of COVID-19
Roche’s Faricimab: First ophthalmic bispecific antibody
Roche’s Faricimab: First ophthalmic bispecific antibody. The first ophthalmic bispecific antibody: Roche’s faricimab in the treatment of DME and nAMD Phase-3 clinical trials: the effect is comparable to Eylea, the treatment interval is longer!
Roche recently announced the detailed results of four global Phase 3 studies evaluating the bispecific antibody faricimab in the treatment of diabetic macular edema (DME) and neovascular or “wet” age-related macular degeneration (nAMD). These studies consistently show that compared with Eylea (aflibercept, aflibercept) once every 2 months dosing schedule, faricimab once every 4 months dosing schedule shows non-inferiority in terms of visual gain. In the first year of the 2 studies for the treatment of DME (YOSEMITE and RHINE) and the 2 studies for the treatment of nAMD (TENAYA and LUCERNE), about half of the patients eligible to extend the administration of faricimab were able to receive treatment every 4 months.
Faricimab is the first injectable eye medicine to achieve this dosing interval (once every 4 months) in a phase 3 study for the treatment of DME and nAMD. In addition, during the first year, approximately three-quarters of patients eligible for extended faricimab administration were able to receive treatment every 3 months or longer. In all 4 studies, faricimab was generally well tolerated, and no new or unexpected safety signals were found.
The detailed results of the 4 Phase 3 studies have been announced at the 18th American “Angiogenesis, Exudation, and Degeneration” Annual Conference (Angiogenesis, Exudation, and Degeneration 2021) to be held on February 13, 2021. The results of all 4 studies will be submitted to regulatory agencies around the world, including the US Food and Drug Administration (FDA) and European Medicines Agency (EMA), to consider regulatory approval of faricimab for the treatment of DME and nAMD.
Jeffrey Heier, MD, director of retinal research at the Boston Eye Consultation Center, said: “These faricimab research data provide a new treatment method for two common causes of blindness (DME and nAMD). The potential of faricimab to extend the treatment interval may help Those who have difficulty keeping up with regular doctor visits and eye injections to maintain vision.”
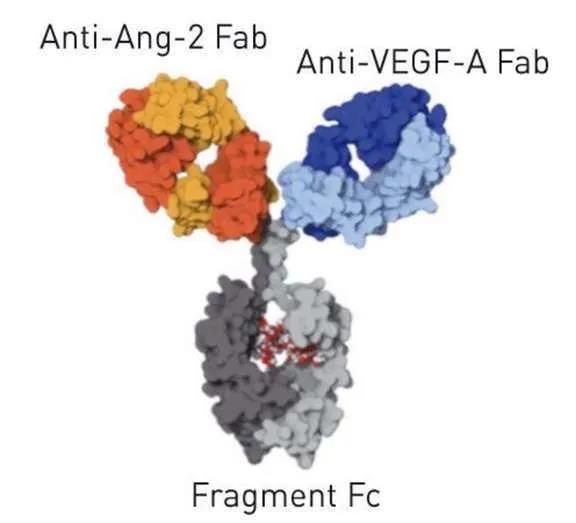
Faricimab structure (picture source: cahiers-ophtalmologie.fr)
Although anti-vascular endothelial growth factor (VEGF) single-agent injections significantly reduce vision loss in patients with DME and nAMD, the treatment burden associated with frequent eye injections and doctor visits may lead to insufficient treatment and may lead to less than optimal vision result.
Faricimab is the first bispecific antibody specifically designed for eyes. Unlike the current DME and nAMD treatments that inhibit the VEGF pathway, faricimab targets two different pathways-through angiopoietin-2 (Ang-2) and vascular endothelial growth factor A (VEGF-A), these two pathways Can drive a variety of retinal diseases, including nAMD and DME. Ang-2 and VEGF-A destabilize blood vessels, cause new leaky blood vessels to form, and increase inflammation, which leads to decreased vision. By blocking these two pathways at the same time, faricimab aims to stabilize blood vessels and reduce inflammation and leakage more than inhibiting either pathway alone. This may improve vision better than anti-VEGF therapy alone, thereby reducing the frequency of eye injections required.
Levi Garraway, MD, Roche’s Chief Medical Officer and Head of Global Product Development, said: “These positive results show that faricimab has the potential to become the first treatment for neovascular age-related macular degeneration (nAMD) and diabetic macular degeneration in the past 10 years. A new drug for edema (DME). This is an exciting time for our ophthalmology clinical development plan. The 2 drugs in our late-stage product line have achieved multiple phase 3 successes. We hope to bring these potentials as soon as possible The treatment method brought to patients with retinal diseases.”
Diabetic macular edema-DME (Image source: bceye.com)
YOSEMITE and RHINE are two global phase 3 studies of the same design, carried out in patients with diabetic macular edema (DME), and evaluated two dosing regimens of faricimab (administered once every 2 months, or as long as every 4 The personalized dosing interval [PTI] of monthly dosing is compared with Eylea’s dosing schedule every 2 months. Patients in the PTI group can receive faricimab treatment every 1 month, 2 months, 3 months, and 4 months, and adjusted according to their disease activity.
Both studies reached the primary endpoint: faricimab has consistently shown non-inferiority compared with Eylea in improving vision. In the YOSEMITE study, the average visual acuity increases in the faricimab PTI group and the 2-month group were +11.6 and +10.7 eye diagram letters, respectively, and the Eylea group was +10.9 letters. In the RHINE study, the average visual acuity increases in the faricimab PTI group and the 2-month group were +10.8 and +11.8 letters, respectively, and the Eylea group was +10.3 letters.
The secondary endpoint of the two studies was to measure the proportion of patients in the faricimab PTI group who achieved a dosing schedule every 3 months or every 4 months at the end of the first year. Importantly, 52.8% (n=151/286) in the faricimab PTI group in the YOSEMITE study and 51% (n=157/308) in the faricimab PTI group in the RHINE study reached every 4 months in the first year Dosing plan.
In addition, 21% (n=60/286) and 20.1% (n=62/308) patients in the faricimab PTI group of the YOSEMITE study and the RHINE study reached the dosing schedule every 3 months. Together, more than 70% of patients in the faricimab PTI group were able to persist for 3 months or more between two treatments at the end of the first year. In these two studies, faricimab administered every 4 months showed a greater reduction in central subzone thickness (CST) compared to Eylea administered every 2 months.
Neovascular age-related macular degeneration (nAMD, image source: researchgate.net)
TENAYA and LUCERNE are two global phase 3 studies with the same design, which were carried out in patients with neovascular age-related macular degeneration (nAMD). Faricimab was evaluated every 2, 3, and 4 months (according to the first 20 weeks and 24 weeks disease activity assessment) dosing schedule, and compared with the Eylea dosing schedule every 2 months.
Both studies reached the primary endpoint: faricimab has consistently shown non-inferiority compared with Eylea in improving vision. In the TENAYA and LUCERNE studies, the average visual acuity increase in the faricimab group compared with baseline was +5.8 and +6.6 letters, respectively, and the Eylea group was +5.1 and +6.6 letters, respectively.
Two studies also measured the proportion of patients receiving faricimab every 3 or 4 months in the first year. Importantly, 45.7% (n=144/315) in the TENAYA study and 44.9% (n=142/316) in the LUCERNE study were able to receive faricimab every 4 months in the first year. In addition, 34% (n=107/315) in the TENAYA study and 32.9% (n=104/316) in the LUCERNE study were able to receive faricimab treatment every 3 months.
Together, nearly 80% of patients in the faricimab treatment group were able to adhere to treatment intervals of 3 months or longer in the first year. In these two studies, treatment with faricimab administered every 4 months showed a greater reduction in central subzone thickness (CST) compared with Eylea administered every 2 months.
(source:internet, reference only)
Disclaimer of medicaltrend.org



