mRNA nucleic acid antibody has a bright future
- Why Lecanemab’s Adoption Faces an Uphill Battle in US?
- Yogurt and High LDL Cholesterol: Can You Still Enjoy It?
- WHO Releases Global Influenza Vaccine Market Study in 2024
- HIV Infections Linked to Unlicensed Spa’s Vampire Facial Treatments
- A Single US$2.15-Million Injection to Block 90% of Cancer Cell Formation
- WIV: Prevention of New Disease X and Investigation of the Origin of COVID-19
mRNA nucleic acid antibody has a bright future
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- What is the difference between Atorvastatin and Rosuvastatin?
- How long can the patient live after heart stent surgery?
mRNA nucleic acid antibody has a bright future.
Describe how mRNA drugs solve the production of antibody and protein drugs, the pain points encountered in the delivery process, and the potential of mRNA drug delivery systems. At the same time, pay attention to the problems and difficulties faced by mRNA drugs in the process of realizing protein drug substitution.
Abstract
Monoclonal antibody technology was founded in 1975. Since then, a steady stream of monoclonal antibody production has become possible. Two scientists, Milstein and Kohler, also won the Nobel Prize in Physiology and Medicine in 1984. In 1986, the world’s first monoclonal antibody drug OKT3 was born.
As of February 2021, 100 antibody drugs have been approved by the FDA for listing. In 2019, the sales of antibody drugs exceeded 140 billion U.S. dollars. In particular, therapeutic antibody drugs represented by immune checkpoints have brought revolutionary treatment options to cancer patients. Immune checkpoint antibodies have also become the most important and successful drugs for cancer treatment.
Although monoclonal antibody pharmaceutical companies have become the fastest-growing companies among pharmaceutical companies, necessary activities such as technology, supervision, and production strategies are still facing huge challenges in the production and preparation of huge clinical antibody demand. This huge challenge comes from the fact that most of the antibody drugs currently used for treatment come from the production of mammalian cells, and a lot of purification work and formulation development work are required in the later stage.
In addition, monoclonal antibodies have a variety of post-translational modifications during the production process, including glycosylation, deamidation, oxidation, mismatches of free cysteine to form additional disulfide bonds, and N-terminal coke valleys. Cyclic structure formed by aminoacylation, C-terminal Lys deletion and other modifications.
These modifications strongly affect the activity and safety of antibodies. Therefore, antibody drug development requires a series of strict characterization and quality control during the production process, and expensive late-stage development is required before it can enter the clinic. In addition, antibody drugs usually only work with proteins on the surface of the cell membrane or outside the cell, which limits their application. Among them, the production of high-quality antibody drugs is not an easy task.
In the past ten years, the research of mRNA medicine has made great progress, which can be used to express any meaningful protein in the body. Currently, a variety of clinical trials are proceeding in an orderly manner, aiming to explore the use of mRNA drugs and disease prevention and treatment.
This article focuses on how mRNA drugs solve the production of antibody and protein drugs, the pain points encountered in the delivery process, and the potential of mRNA drug delivery systems. At the same time, pay attention to the problems and difficulties faced by mRNA drugs in the process of realizing protein drug substitution.
Background
The research and discovery of nucleic acid drugs has gone through a long journey.
▨ mRNA was discovered in 1961 as a carrier of genetic information transmission. It is transcribed from DNA and directs the translation of proteins;
▨ In 1978, Harvard University scientists discovered that a complementary nucleotide chain can inhibit the replication of RSV virus, which is the concept of ASO (antisense oligonucleotide, antisense nucleic acid);
▨ In 1990, Science and Nature respectively reported that RNA strands with strong affinity to target protein molecules were screened out in vitro, that is, RNA aptamer or RNA aptamer; in the same year, it was discovered that injecting mRNA into mice can make the corresponding expression.的protein;
▨ In 1998, the first ASO drug was approved; in the same year, the mechanism of RNAi (RNA interference) was revealed;
▨ In 2004, the first RNA aptamer drug was approved; in 2018, the first RNAi-based drug siRNA (small interfering RNA) was approved.
The research and development of nucleic acid drugs has experienced a trough. Nucleic acid drugs suffered repeated setbacks due to immunogenicity and delivery system issues around 2010. Novartis and Roche suspended their cooperation with Alnylam. Pfizer and Abbott suspended the RNA drug research project. In 2012, EMA refused due to liver and cardiovascular side effects. After Mipomersen’s listing application, MSD sold Sirna to Alnylam in 2014 at a price lower than the original purchase price.
Breakthroughs in key technologies have promoted the development of nucleic acid drugs. The more important ones are the chemical modification of nucleotides and the application of delivery systems. Chemical modification of nucleotides can improve the stability of nucleic acid molecules and reduce their immunogenicity. The development of delivery system technology is making it possible to prevent nucleic acid drugs from being The nuclease degrades at the same time to increase the efficiency of its entry into the cell. Breakthroughs in key technologies and continuous research have enabled the nucleic acid drug industry to continue to flourish.
siRNA is one of the research hotspots in the field of nucleic acid drugs. Since the mechanism of RNAi was revealed in 1998, the development of RNAi drugs has undergone rapid development in the past 20 years. It can induce gene silencing, so it is called siRNA, usually 20-25 pairs of nucleotides in length. In 2018 and 2019, 1 siRNA drug was approved, and 2 siRNA drugs were approved in 2020. At the same time, there are many siRNA drugs in clinical development.
mRNA has received a lot of attention in the COVID-19 epidemic. At present, the two nucleic acid COVID-19 vaccines authorized by the US FDA for EUA are both mRNA vaccines. Theoretically, mRNA has the potential to synthesize any kind of protein, so mRNA can be used as a protein supplement or replacement therapy to treat a variety of diseases; and one mRNA can express multiple proteins to achieve combination medication; after the target is confirmed, mRNA drugs/ The design and discovery of vaccines is very fast.
Exogenous nucleic acid drugs need to overcome numerous obstacles in order to enter the body to play a role. These obstacles have also caused difficulties in the research and development of nucleic acid drugs. However, with the development of new technologies, some problems have been solved well.
▨ Nanoparticle structure package prevents mRNA from being quickly cleared and prolongs the half-life;
▨ Chemical modification of nucleotides to avoid degradation by nucleases;
▨ Nanoparticle structure optimization improves the permeability of nucleic acid drugs in tissues;
▨ Modification of the delivery system to improve the efficiency of binding to target cells and cell uptake;
▨ Including peptides, polymers, and ionizable lipids to improve the escape efficiency of endosomes.
The first entry field of mRNA therapy is the field of therapeutic cancer vaccines, which requires a lower safety standard. With the further optimization of IVT mRNA and the further reduction of inflammatory side effects, IVT mRNA has entered the second field of application, that is, the field of vaccination. There are different clinical studies passed and ongoing IVT mRNA in the field of cancer vaccination and vaccination.
Outside of these two areas, people are interested in IVT mRNA as a protein replacement therapy. However, this is very challenging because it requires targeted expression of mRNA and repeated administration, and in some cases even systemic administration. These requirements mean that the system must have high security, which is very challenging. Recently, mRNA therapy has also entered the field of gene editing.
Most of the research and development of mRNA is concentrated in the field of vaccines for several reasons:
▨ Compared with vaccines, protein replacement therapy requires higher protein expression, and vaccines only need a relatively small amount of antigen expression to activate the human immune system;
▨ Protein replacement therapy usually requires continuous protein expression, so frequent administration may be required, while vaccines only need a few times;
▨ The mRNA molecule itself is immunogenic, which is more conducive to the effect of the vaccine;
▨ Protein replacement therapy requires the correct post-translational modification of the protein. The modification process may have different results in different cells. Therefore, it is necessary to deliver mRNA to the correct cell to achieve better results. At present, targeted delivery is still difficult to achieve .
In addition to vaccines, mRNA as a protein replacement therapy is also being explored. Especially the use of mRNA encoding antibodies for immunotherapy, many products have entered the preclinical or clinical stage; compared to the one-time administration of each injection of antibodies, the translation of mRNA after entering the human body can last for several days, so it is expected to reduce the administration Frequency; and mRNA can enter the cell, so it can encode antibodies against intracellular targets; at the same time, mRNA can encode a variety of antibodies, and it is easier to produce mRNA that can encode multiple antibodies than to directly produce multiple antibodies.
Origin of mRNA antibody
Hoerr et al. first proposed the concept of using mRNA to encode antibody protein in a patent application in 2008, and RNA encodes antibody (EP 2101823 B1). In March 2017, Pardi et al. published the first article on the feasibility of using mRNA for passive vaccination.
In this article, m1ψ contains the mRNA encoding the light and heavy chains of VRC01 (a broadly neutralizing antibody against HIV-1), which is administered systemically in the form of liposomal nanoparticles, LNP. Pardi et al. also confirmed that intravenous injection of the liposome nanoparticles can efficiently express the target protein in the liver.
A single intravenous injection of 30 μg of mRNA-LNP expressing VRC01 antibody, the level of VRC01 in serum reached a peak at 24 hours, and gradually decreased over time, until the next injection on the 11th day. MRNA-LNP encoding VRC01 antibody is better than direct administration of VRC01 antibody in the prevention of HIV-1 in mouse models.
A few months later, Thran and others confirmed the feasibility of using mRNA for passive vaccination in three different disease models: as an anti-pathogen therapy (rabies model), as an antitoxin therapy (botulism model), and as an anti-pathogen therapy (rabies model). Anti-tumor therapy (lymphoma model).
Experiments have shown that a single injection of mRNA-LNP encoding monoclonal antibodies or camel heavy chain antibodies (VHHs) is sufficient to establish rapid, potent and durable serum antibody titers in the body. These high-titer antibodies can completely protect mice from virus attack or poisoning, and can eliminate tumor cells in mouse models.
In addition, the researchers also studied the general tolerance of mRNA-LNP treatment. Although some circulating cytokines have short-term low-level increases, this weak increase does not prevent the expression of high protein. More importantly, the histopathology of the liver, the target organ of mRNA-LNPs, did not reveal any signs of abnormality or inflammation.
In the same period, the third researcher Stadler et al. reported on the preclinical study of mRNA used in the construction of bispecific antibody BiTE. Shows a strong anti-tumor effect. Compared with monoclonal antibody drugs, traditional bispecific antibody drugs have a more complicated and tedious process of expression and purification.
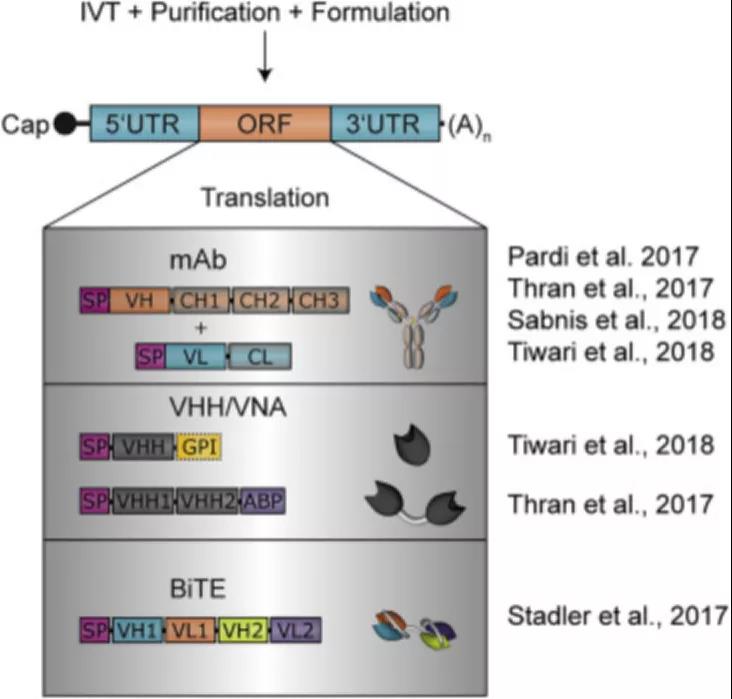 Figure 1: Overview of nucleic acid antibody design
Figure 1: Overview of nucleic acid antibody design
In order to solve these problems, Stadler et al. designed IVT-modified mRNA, which encodes bispecific antibodies, called RiboMABs, which target the T cell receptor-related molecule CD3 and one of the three tumor-associated antigens (TAA). Compared with traditional protein-based bispecific antibodies, Ribomab is easier to use and requires a smaller dose to produce Abs that achieve therapeutic effects.
But the main advantage is that in terms of development, you can easily make the corresponding RNA by changing the DNA and compare it with the candidate antibody structure. This rapid procedure allows the evaluation of different antibodies in a short period of time.
A few micrograms of mRNA encoding RiboMABs can be injected intravenously to achieve rapid expression of antibodies in the liver and circulate into the bloodstream. The level of RiboMABs in the blood reaches its peak within a few hours, and can maintain a therapeutic level concentration for about a week.
Experiments have shown that RiboMABs are tested in xenograft mouse models with larger ovarian tumors. The 3-week RiboMAB treatment completely eliminated the tumor, which was comparable to the effectiveness of the corresponding recombinant bispecific antibody, although the latter required three uses to achieve the same degree of tumor eradication.
The studies discussed above require the liver to act as a bioreactor to translate mRNA and provide antibodies systematically to deliver mRNA through the vein. The difference is that Tiwari et al. reported the local delivery of palivizumab and VHH mRNA encoding anti-respiratory syncytial virus (RSV) monoclonal antibody (palivizumab).
Since protection from respiratory syncytial virus infection only requires protective antibodies in the lungs, rather than the entire body, local delivery of mRNA encoding Ab is more advantageous. The authors used secretory and membrane-anchored palvizumab or anti-rsv VHH encoded by naked mRNA to deliver them to the lungs through intratracheal aerosol.
Studies have shown that using this delivery method, as many as 45% of lung cells display detectable antibody expression, resulting in RSV infection, secreted Ab can be maintained for 4 days, and anchored VHH can be maintained for 7 days. Virus infection is greatly reduced. More importantly, the naked mRNA is delivered through tracheal aerosol, and no significant increase in cytokine expression is observed in the lungs within 24 hours after treatment.
Difficulties and challenges of encoding antibody mRNA
The instability of mRNA is that the idea of its application in treatment is full of challenges. In order to improve molecular stability and protein translation, the researchers made a series of modifications to the vector used to produce mRNA and the synthetic mRNA itself.
The mRNA in vitro transcription template consists of five cis-acting structural elements, from 5’ to 3’ as follows:
▨ Optimized hat structure;
▨ Optimized 5’UTR;
▨ Optimized target gene sequence;
▨ Optimized 3’UTR;
▨ Optimized polyadenylic acid polyA tail. These cis-acting structural elements are continuously optimized to obtain better mRNA characteristics.
The poly-A-tail and cap structures are of great significance to the effective translation of mRNA and stable mRNA decay, while UTR controls the translation and half-life of mRNA. Finally, purification and incorporation of modified nucleosides, including 1-methylpseudouracil (m1ψ), such as high performance liquid chromatography, makes mRNA immunogenicity non-immunogenic and significantly improves translation efficiency.
So far, a variety of products encoding antibody mRNA have entered the preclinical or clinical stage, and this expression and effectiveness need to be further verified on the human body. Earlier studies on another type of secreted protein, erythropoietin, showed that the findings in mouse models can be applied to large animals, such as domestic pigs, and even primates. These results give us hope that the data of mRNA-encoded antibodies in the mouse model may also be analogous to humans.
Three principles for the feasibility of monoclonal antibody pharmacy:
▨ Serum antibody titer increases rapidly after injection;
▨ The serum antibody of Emperor Capital is high enough to play a protective and therapeutic role;
▨ The half-life of serum antibodies is long enough. The increase and level of monoclonal antibodies depends on the formulation and delivery route of the drug.
Most of the mRNA delivery methods that have been developed are based on the formation of nanoparticles, such as the use of liposomes, polymers, and peptides. Pardi et al. found that mRNA encapsulated in lipid nanoparticles can produce high levels of protein for a longer period of time when administered through multiple routes. So far, only lipid nanoparticle vectors have been used for mRNA encoding Ab. In addition to the form of mRNA, the delivery system also affects the titer of serum antibodies.
There are three main delivery routes for mRNA vaccines currently studied: local delivery (eg, intrapulmonary, intradermal and subcutaneous), targeted delivery (lymph nodes) or systemic delivery (venous). Recent studies have shown that antibody titers can be detected on the first day after intravenous injection of mRNA encoding antibodies. So far, only studies by Tiwari et al. have shown data on local delivery of intratracheal aerosols to the lungs. Except for these two methods of administration, no other routes of administration have been detected with mRNA encoding Abs.
Given the approved treatment regimens, it is very important to target mRNA delivery to the organ of interest to minimize the amount of drug required to cause systemic toxicity, anti-antibody immune response, and reach therapeutic levels. Moreover, targeted delivery may also reduce the amount of mRNA reaching the target cell, thereby reducing the antibody encoded by the cell.
The therapeutic doses of recombinant antibodies currently used are usually very high. So far, it is not clear whether these high doses can be achieved by the administration of encoding mRNA. Despite this, there are still people who are optimistic about the use of mRNA as a therapeutic drug over recombinant antibodies.
▨ First of all, because the antibody is expressed in situ, it reduces the local high protein concentration to achieve the therapeutic effect.
▨ Secondly, based on the doses tested so far, no saturation or dose-limiting toxicity of mRNA-mediated antibodies has been detected.
▨ Furthermore, optimizing the targeted mRNA and further improving the formula may further greatly improve the efficacy.
The serum half-life of the Ab encoded by the mRNA depends on the half-life of the Ab itself on the one hand, and on the mRNA encoding the Ab on the other hand. More specifically, the half-life of the first stage is determined by the half-life of mRNA and protein, while the half-life of the second stage is almost entirely determined by the characteristics of the protein.
This means that the half-life of short-lived proteins can significantly benefit from mRNA expression. For long-lived proteins, using the mRNA expression platform has no significant effect on the duration of the therapeutic effect, but the mRNA half-life does contribute to peak expression. In addition, the size of the antibody molecule limits its applicability in mRNA format. For example, the commonly used IgG subtype is in the range of 150 kDa.
In addition, antibodies are complex multi-domain proteins that must be assembled in the correct way. In order to overcome the size and stability limitations of mRNA-encoded monoclonal antibodies, a lot of research has been conducted on heavy chain antibodies (VHH) and small non-antibody scaffold proteins.
There are two types of non-antibody scaffolds, namely (i) domain-sized compounds (6 to 20 kDa), such as DARPins alphabodies, and (ii) constrained peptides (2-4 kDa). Different stents are currently in academic research, preclinical and clinical development stages, and have shown great potential in terms of affinity, target neutralization and stability.
However, non-antibody scaffolds also face their own challenges, in which serum half-life and tissue penetration are the most important. It is particularly worth studying the effectiveness of mRNA encoding for non-antibody scaffolds, because the mRNA platform can have a positive effect on serum half-life, as described above.
In order to avoid complicated production and purification processes and abnormal post-translational modifications of protein-based monoclonal antibodies, alternative methods for rapid production, management and testing of monoclonal antibodies are currently being explored. Nucleic acid therapy has great potential because it is simple, fast, and cost-effective, does not require complex and expensive laboratory infrastructure, and all mRNA has a common production process. Recent advances in mRNA, including improvements in in vitro transcription, have increased interest in the therapeutic potential of this biomolecule.
Unlike DNA, mRNA only needs to reach the cytoplasm to induce protein expression, and there is no obvious risk of insertion mutation. Compared with the protein-based platform, the mRNA-based Ab therapy platform has different advantages.
▨ First, the expression of Ab encoded by mRNA can be detected within a few days.
▨ Secondly, the mRNA form is easier to produce intracellular antibodies.
▨ Third, since protein is composed of 20 different amino acids, the physical and chemical properties of different proteins are different, which means that the storage buffer and formula of each protein need to be optimized separately.
mRNA only uses four nucleosides, resulting in its structure basically having the same physicochemical characteristics, regardless of the physicochemical properties of the protein it encodes. A strategy based on mRNA encoding an antibody has been reported, and this strategy has gradually matured over the past few decades.
So far, the applicability of the mRNA platform for antibody therapy has been studied in the context of antitoxins, infectious diseases and oncology. Although initial reports indicated that mRNA is an emerging antibody gene transfer platform, the further development of mRNA-based mAbs is limited by the need for safe and effective delivery systems.
In addition, mRNA can only produce post-translational modifications of the antibody in its natural state. The strategy of increasing the serum half-life of antibodies by modification cannot be realized on the platform of mRNA-encoded antibodies.
For passive immunity, very high safety is required. In the past few decades, people have performed different optimizations on IVT mRNA to avoid harmful immune activation and cytokine induction of cellular RNA recognition receptors. ADA (anti-drug antibody) response and transient cytokine emergence are still It can be detected, thus hindering the clinical applicability of mRNA drugs, especially when multiple high-dose administrations are required.
It is worth noting that when nanoparticles are repeatedly administered, they can also induce pseudoallergic complement activation. Since mice are not sensitive to induced complement activation pseudoallergies, more careful analysis of the formulation is required.
Research examples
Recently, a group of scientists have designed a brand-new therapy, which is expected to introduce mRNA encoding effective antibodies into animals, and synthesize antibodies in situ to treat diseases. This innovative breakthrough was published in Science Immunology, a sub-issue of “Science”.
mRNA-encoded Nanobodies and their applications, especially in the field of Nanobodies medicine. In order to solve the above problems, a new idea and method system for regulating intracellular proteins with RNA-encoded Nanobodies. A nanobody encoded by mRNA, the encoded information carried by the mRNA is recognized, translated, and expressed in the cell to produce a single-chain nanobody that can bind to the target protein.
The method can effectively and specifically interfere with the function of the disease-related proteins in the cell, and thus achieve the purpose of treating the disease. At the same time, this method is different from the commonly used strategy of expressing Nanobodies in cells using DNA or virus as a vector. This method eliminates the risk of altering the host cell genome sequence, and is safe and easy to remove. It is very suitable for clinical drug design and production. need
The disease targeted in this study is “chikungunya virus” (CHIKV) infection. Although this is not as widely concerned as cancer or Alzheimer’s disease, it represents a huge unfinished medical demand. In the absence of existing treatments, patients can only rely on their own immunity if they want to overcome this disease.
The researchers found a patient who was self-healing and analyzed the B cells in his body. This analysis found a series of powerful neutralizing monoclonal antibodies against CHIKV infection. Among them, a monoclonal antibody called CHKV-24 has the strongest anti-infection ability in vitro.
Subsequently, the researchers confirmed that this human-derived antibody can also protect mice. 24 hours after the injection of purified antibodies, the researchers injected them with a lethal amount of virus. The results showed that the mice that received high dose (10 mg/kg) and medium dose (2 mg/kg) of CHKV-24 antibody had a survival rate of 100%! The mice that received the lowest dose (0.4 mg/kg) of the antibody also had a survival rate of 50%. These results indicate that the antibody isolated from human patients can indeed protect mice.
In order to understand whether the mRNA encoding the CHKV-24 antibody can also play a protective role, the researchers obtained the sequence of the mRNA and used lipid nanoparticles to encapsulate the mRNA. After being injected into mice, these mRNAs can successfully function and express human CHKV-24 antibodies.
Subsequently, the researchers used immunodeficient mice for testing. Under normal circumstances, these mice are extremely sensitive to CHIKV infection and are difficult to survive. When injected into the mRNA encoding the CHKV-24 antibody, these mice really developed resistance to the lethal amount of the virus, and the resistance became stronger with the increase of the mRNA-in the highest dose group, the 21 infected by the virus Days later, the survival rate of mice still remains 100%! In the control group, all the mice died after only 4 days! At present, a phase 1 human clinical trial has been officially launched to evaluate the safety and tolerability of this mRNA therapy.
“Using the body’s own mechanism to produce antibodies and using mRNA to produce antibodies against CHIKV is expected to be a powerful method to combat this disease,” said Professor James Crowe, the corresponding author of this study. “We are very pleased to see that early work can help. We have made contributions to pre-clinical research, and we look forward to seeing the results from the phase 1 clinical trial.”
Application of nucleic acid-encoded antibodies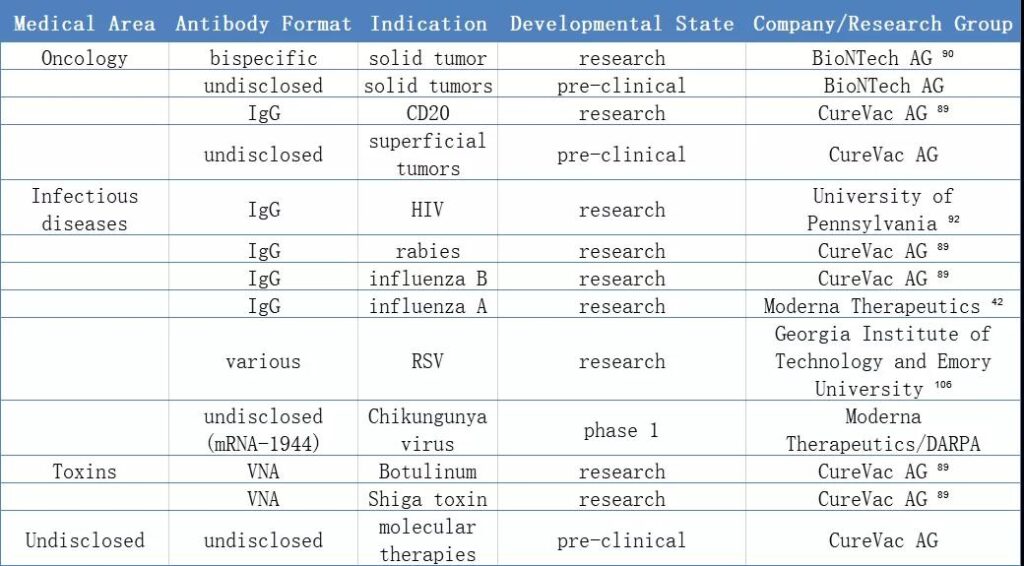
Conclusion
Antibodies have shown unique curative effects in the fields of anti-infection, cancer treatment, respiratory diseases, metabolism, and cardiovascular diseases. The time, money, and purification of antibody production, and the complicated downstream processes have to make people try more possibilities.
Encoding antibody mRNA is a viable treatment option, which can avoid complicated production and purification processes and the inherent abnormal post-translational modification problems of protein mAbs.
Nevertheless, to successfully replace the current protein antibody format, mRNA methods need to surpass their own challenges, which are mainly drug delivery and mRNA immunogenicity levels. However, with the emergence of mRNA as a treatment method and the continuous growth of research on this topic, the therapy of mRNA encoding antibody is likely to be further developed and improved in the next few years.
mRNA nucleic acid antibody has a bright future
mRNA nucleic acid antibody has a bright future
mRNA nucleic acid antibody has a bright future
mRNA nucleic acid antibody has a bright future
mRNA nucleic acid antibody has a bright future
mRNA nucleic acid antibody has a bright future
mRNA nucleic acid antibody has a bright future
(source:internet, reference only)
Disclaimer of medicaltrend.org



