IL-12 family (Rheumatism | Tumors): different functions
- Oregon Reverses Course: From Decriminalization to Recriminalization of Drug Possession
- Why Lecanemab’s Adoption Faces an Uphill Battle in US?
- Yogurt and High LDL Cholesterol: Can You Still Enjoy It?
- WHO Releases Global Influenza Vaccine Market Study in 2024
- HIV Infections Linked to Unlicensed Spa’s Vampire Facial Treatments
- A Single US$2.15-Million Injection to Block 90% of Cancer Cell Formation
IL-12 family (Rheumatism | Tumors): different functions
IL-12 family (Rheumatism | Tumors): different functions. The cytokines of the IL-12 family show similarities in structure, but have different functions in the immune system. Among them, the pro-inflammatory cytokines IL-12 and IL-23 have been studied in depth.
These two cytokines share the cytokine subunit p40 and receptor β chain, but have different functions in autoimmune diseases, cancer, and infections.
Stelara/Ustekinumab for IL-12/IL-23 p40 subunit has been approved in 2009 for rheumatic diseases such as psoriasis.
IL-12 family
IL-12 family members and receptors
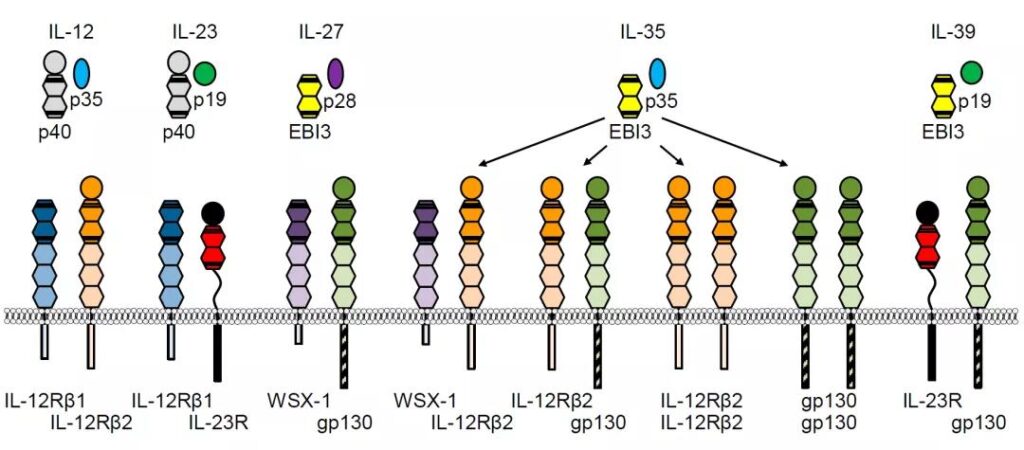
IL-12 family (document 1)
IL-12 and IL-23 share the p40 subunit, the difference is that IL-12 carries p35 and IL-23p19
IL-27, IL-35, IL-39 share EBI3, and then carry p28, p35, p19 respectively
The receptor structure is also different, see the figure above for details.
Similarities and differences between IL-12 and IL-23
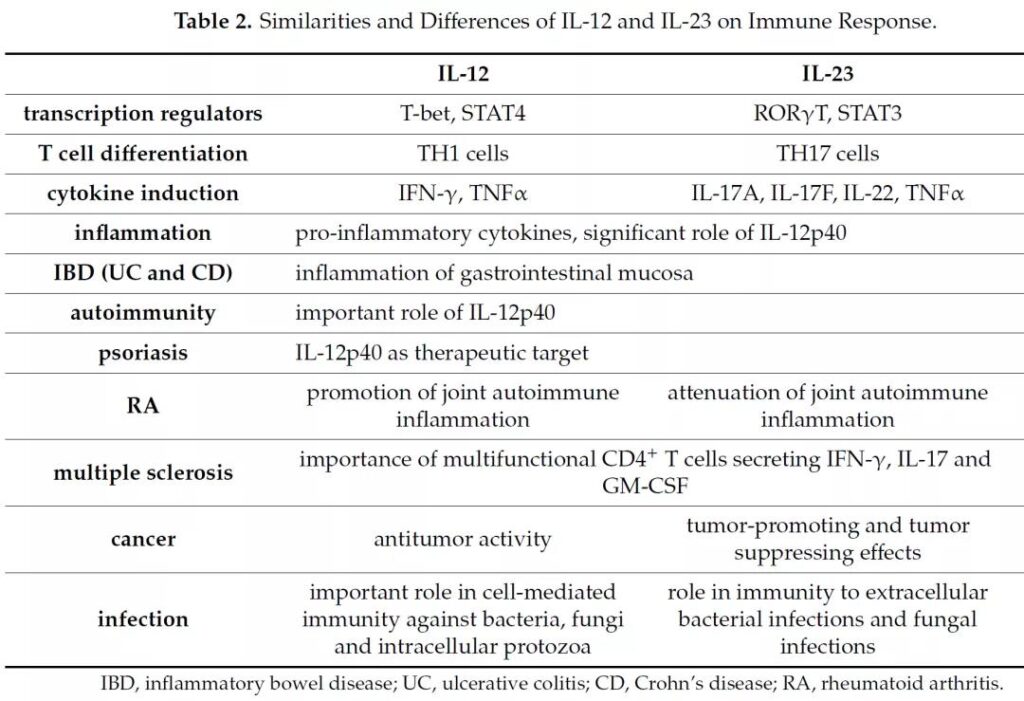
Literature 2
- IL-12 promotes the differentiation of TH1T cells and has strong anti-tumor activity.
- IL-23 promotes the differentiation of TH17 cells and participates in rheumatic diseases.
Rheumatic disease drugs
Stelara/Ustekinumab (Usnumab)
Stelara can inhibit the effects of these two pro-inflammatory cytokines by binding to the p40 subunit shared by IL-12 and IL-23, preventing it from binding to the cell surface receptor IL-12 β1.
Stelara was first approved by the FDA in September 2009 for use in adult patients with moderate to severe plaque psoriasis. The approved indications include: psoriasis; active psoriatic arthritis; Crohn’s disease, etc.
Sales of this product in 2016 were US$3.232 billion, US$4.011 billion in 2017, and US$5.251 billion in 2018.
It has been marketed in China, the product name: Usnumab injection, the product name: Starno.
Registration number: S20170046 (90mg/ml/piece)
Registration number: S20170047 (45mg/0.5ml/piece)
Registration number: S20200005 (130mg/26ml/bottle)
Tremfya/Guselkumab (IL-23 monoclonal antibody)
In July 2017, Guselkumab (trade name: Tremfya) produced by Johnson & Johnson was launched in the United States. Because Guselkumab defeated adalimumab and secukinumab in a head-to-head study, it is known as the “best psoriasis drug”. Indications: Psoriasis.
National Medicine Standard SJ20202004
Product name: Gusaiqi Yumab Injection
Commodity name: Tenuoya
Product specifications: 100mg/1mL/piece (prefilled pen syringe)
Ilumetri/ Tildrakizumab
A humanized monoclonal antibody that selectively and high affinity binds to the p19 subunit of IL-23, inhibits its binding to the IL-23 receptor, thereby inhibiting the release of pro-inflammatory cytokines and chemokines.
Ilumetri is produced by the Spanish pharmaceutical company Almirall and is used to treat adults with moderate to severe plaque psoriasis.
In the European Union, Ilumetri was approved in September 2018 and is currently listed in Germany and will continue to be listed in all EU member states. Ilumetri will provide patients with a simple treatment plan. After completing the initial treatment in the 0th and 4th weeks, during the subsequent maintenance treatment, only subcutaneous injections are required every 3 months, that is, only 4 doses per year, which will provide patients with Greater convenience, better disease control, and improved treatment satisfaction.
Skyrizi/Risankizumab
Risankizumab is produced by AbbVie and obtained a Biologics License Application (BLA) in 2019 for the treatment of patients with moderate to severe plaque psoriasis.
Risankizumab is an IL-23 inhibitor that selectively blocks IL-23 by binding to the p19 subunit of IL-23. In addition to psoriasis, the drug is also evaluated in patients with psoriatic arthritis and Crohn’s disease, and it is planned to be evaluated in patients with ulcerative colitis in the future.
Brazikumab
Allergan started two clinical studies of Brazikuumab in 2018 for the treatment of Brazilian inflammatory bowel disease (IBD) to evaluate the safety and effectiveness of this IL-23 inhibitor treatment.
Mirikizumab
In June 2018, the US pharmaceutical giant Eli Lilly announced at the 2018 Digestive Disease Week (DDW2018) in Washington, the United States that the monoclonal antibody mirikizumab in the treatment of moderate to severe ulcerative colitis (UC) phase II clinical study new Safety and efficacy data. The data showed that in the 12th week of treatment, compared with the placebo group, the mirikizumab treatment group had a significantly higher clinical remission rate.
mirikizumab is a humanized IgG4 monoclonal antibody that targets the p19 subunit that binds IL-23. At present, mirikizumab is being developed for a variety of immune diseases, including psoriasis, UC and Crohn’s disease (CD). Among them, the treatment of moderate to severe plaque psoriasis is in phase III clinical trials.
Ilumya/tildrakizumab
This new drug, produced by Sun Pharma, can selectively bind to the p19 subunit of IL-23 and inhibit its interaction with the IL-23 receptor, thereby inhibiting the release of pro-inflammatory cytokines and chemokines. After completing the initial dose in the 0th and 4th weeks, a 100 mg dose was injected subcutaneously every 12 weeks. Suitable for adult patients with moderate to severe plaque psoriasis receiving systemic therapy or phototherapy.
IL-12 anti-tumor therapy
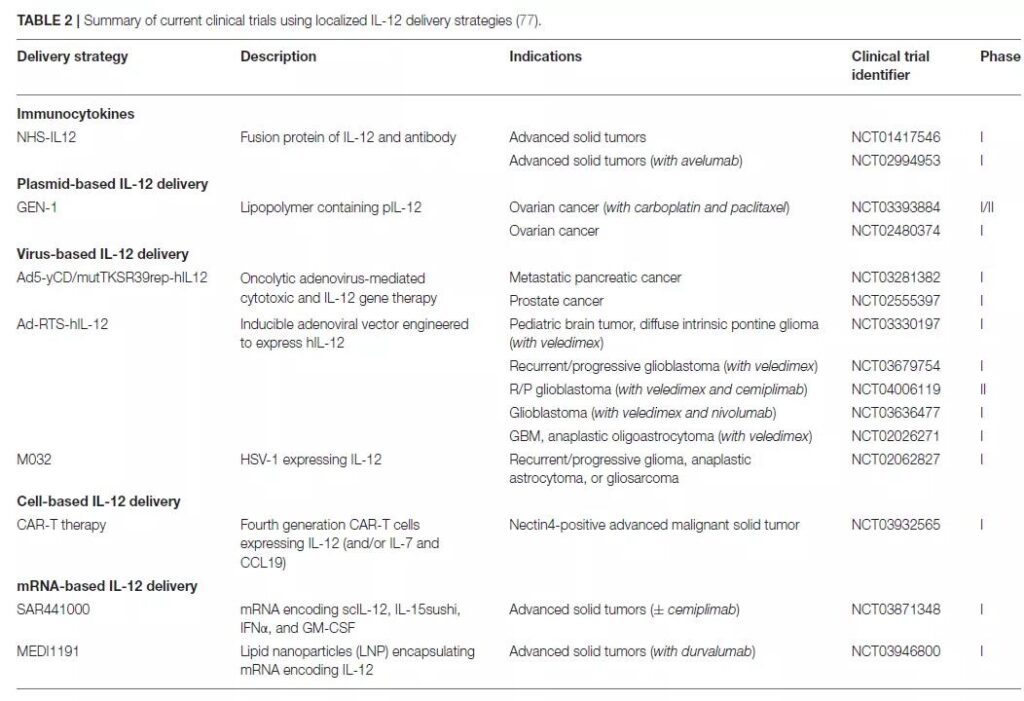
Literature 3
There are more than 600 IL-12 clinical trials registered in ClinicalTrials.gov, of which more than 120 are recruited, involving gene therapy, mRNA vaccines, CAR-T, combination chemotherapy, immune checkpoint inhibitors, etc.
Examples are as follows:
Gene therapy
NCT02026271, R&D unit Ziopharm, A Study of Ad-RTS-hIL-12 With Veledimex in Subjects With Glioblastoma or Malignant Glioma, Phase1
mRNA vaccine combined with PD-L1 monoclonal antibody
NCT03946800, R&D unit MedImmune LLC, A Study of MEDI1191 in Sequential and Concurrent Combination With Durvalumab in Subjects With Advanced Solid Tumors, Phase1
On March 1-2, 2021, the 19th ESMO International Targeted Anti-Cancer Therapy Conference (ESMO TAT) reported preliminary results. Two patients with IL-12mRNA vaccine combined with Durvalumab (anti-PD-L1 monoclonal antibody) developed immunity The response, safety and tolerability were good, and no adverse reactions of grade ≥3 occurred.
IL-12 DNA electrotransfection combined with K drug or O drug
NCT03132675, R&D unit OncoSec Medical Incorporated/Merck Sharp & Dohme Corp. Tavo and Pembrolizumab in Patients With Stage III/IV Melanoma Progressing on Pembrolizumab or Nivolumab Treatment (Keynote695), Phase2
(tavo; pIL-12): intratumoral tavokinogene telseplasmid
Antibody cytokine IL-12 fusion
NCT04471987, Research and Development Unit Philogen S.p.A., Safety and Early Signs of Efficacy of IL12-L19L19. (DODEKA)
Expert Comments:
There are many members in the IL-12 family, among which IL-12 promotes TH1 differentiation and anti-tumor is the main concern. IL-23 promotes the differentiation of TH17, and autoimmune diseases are the focus.
Of course, it is not absolute. For example, it is now believed that Th1/Th17 phenotype plays an important role in rheumatic diseases, so there may be a crossover between them. The remaining cytokines have not been studied very deeply at present and need to be accumulated more in the future.
(source:internet, reference only)
Disclaimer of medicaltrend.org



