Finerenone: Bayer’s New drug medicine of for diabetic nephropathy!
- Why Lecanemab’s Adoption Faces an Uphill Battle in US?
- Yogurt and High LDL Cholesterol: Can You Still Enjoy It?
- WHO Releases Global Influenza Vaccine Market Study in 2024
- HIV Infections Linked to Unlicensed Spa’s Vampire Facial Treatments
- A Single US$2.15-Million Injection to Block 90% of Cancer Cell Formation
- WIV: Prevention of New Disease X and Investigation of the Origin of COVID-19
Finerenone: Bayer’s New drug medicine of for diabetic nephropathy!
Finerenone: Bayer’s New drug medicine of for diabetic nephropathy, succeeded in phase 3 cardiovascular outcome study.
Bayer recently announced the results of its Phase III cardiovascular outcome study FIGARO-DKD.
The study was carried out in patients with both chronic kidney disease (CKD) and type 2 diabetes (T2D), and evaluated the efficacy and safety of finerenone (BAY 94-8862) and placebo.
Both groups received standard care, including hypoglycemic therapy and guidelines-guided maximum tolerated dose of angiotensin-converting enzyme inhibitor (ACEI) or angiotensin II receptor blocker (ARB).
The results showed that the study reached the primary endpoint: when combined with standard care, finerenone significantly reduced the incidence of first cardiovascular (CV) deaths or non-fatal CV events (myocardial infarction, stroke, heart failure hospitalization) compared with placebo Compound risk. The detailed data of the study will be announced at an upcoming medical conference.
FIGARO-DKD is the second positive phase 3 study in the phase 3 clinical program of finerenone for the treatment of CKD and T2D patients. Compared with FIDELIO-DKD, the first positive phase 3 study in the project, the FIGARO-DKD study enrolled more patients with early-stage CKD and T2D. The previously published FIDELIO-DKD study showed that when combined with standard care, finerenone significantly reduced the risk of the composite primary endpoint of CKD progression, renal failure, and renal death compared with placebo.
Professor Luis M. Ruilope of the Department of Public Health and Preventive Medicine of the Autonomous University of Madrid, Spain, said: “Up to 40% of patients with type 2 diabetes will develop chronic kidney disease, and they are very likely to have cardiovascular events. And progress to renal failure. The FIGARO-DKD study provides important insights into the potential impact of finerenone on cardiovascular outcomes in patients with chronic kidney disease and type 2 diabetes.”
Dr. Christian Rommel, Member of the Executive Committee and Head of R&D of Bayer Pharmaceuticals, said: “With the positive results of the FIGARO-DKD study at the composite primary endpoint, by completing the largest CKD and T2D phase III clinical trial project to date covering a wide range of disease severity, We have achieved an important milestone in the clinical development of finerenone. We are very pleased to see that the data from the FIGARO-DKD study further supports the evidence generated by the FIDELIO-DKD study, reducing cardiovascular deaths and non-fatal cardiovascular deaths in patients with CKD and T2D Compound risk of event or outcome.”
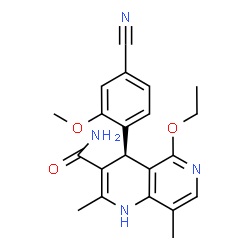
The chemical structure of finerenone (picture source: newdrugapprovals.org)
Chronic kidney disease (CKD) is one of the most common complications of diabetes and an independent risk factor for cardiovascular disease. In all patients with type 2 diabetes, approximately 40% of patients will develop CKD. CKD is the main cause of end-stage renal disease and renal failure. In the advanced stage, patients may require dialysis or kidney transplantation to survive.
In 10 years, type 2 diabetes patients with CKD are three times more likely to die from cardiovascular-related diseases than patients with type 2 diabetes alone. It is well known that in patients with CKD and type 2 diabetes, excessive activation of mineralocorticoid receptors can trigger harmful processes (for example, inflammation and fibrosis) in the kidneys and heart. Globally, CKD in patients with type 2 diabetes is the most common cause of renal failure.
Finerenone is a pioneering, non-steroidal, selective mineralocorticoid receptor antagonist (MRA) that has been shown to reduce the harmful effects of excessive mineralocorticoid receptor (MR) activation. Excessive activation of mineralocorticoid receptors is the main driver of kidney and heart damage. Finerenone has been shown to have positive renal and cardiovascular benefits in patients with CKD and T2D.
At present, Finerenone is undergoing review by US, EU, and Chinese regulatory agencies. It is worth mentioning that finerenone is the first non-steroidal selective mineralocorticoid receptor antagonist (MRA) that has been proven to reduce the risk of renal and cardiovascular events in patients with T2D and CKD. Although progress has been made in recent years, many patients with CKD and T2D are still moving towards end-stage renal failure or premature death. The mechanism of action of finerenone is different from current treatments. If approved, the drug can slow the progression of the disease by directly targeting inflammation and fibrosis (the main drivers of CKD progression).
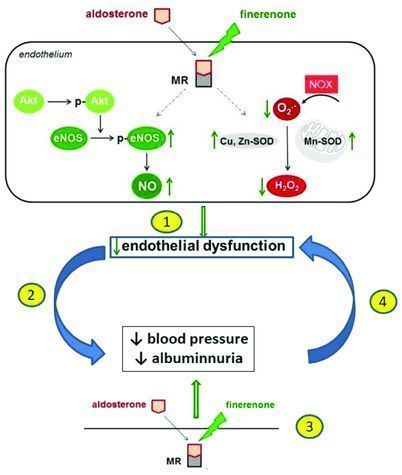
The mechanism of finerenone (picture source: researchgate.net)
The finerenone phase III clinical project is the largest CKD combined T2D phase III clinical project to date. The project consists of 2 studies and enrolled 13,000 T2D patients with widespread CKD disease from all over the world, including patients with early renal damage and more advanced renal disease. This project aims to evaluate the effects of finerenone and placebo in combination with standard care on the prognosis of kidney and cardiovascular (CV).
The phase 3 FIDELIO-DKD study (finerenone reduces renal failure and disease progression in diabetic nephropathy) enrolled approximately 5,700 patients, and the phase 3 FIGARO-DKD study (finerenone reduces the incidence and death of cardiovascular disease in diabetic nephropathy) enrolled approximately 7,400 patients patient.
Finerenone’s regulatory application documents are based on the positive data of the FIDELIO-DKD study. The results of the test were announced at Kidney Week Reimagined 2020 (ASN) and published in the New England Journal of Medicine (NEJM) in October 2020. See: Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes.
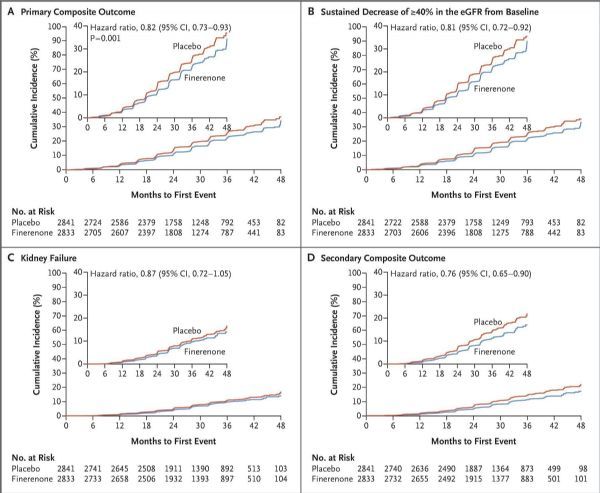
FIDELIO-DKD research data (picture)
The FIDELIO-DKD study was carried out in patients with CKD and T2D to evaluate the efficacy and safety of finerenone and placebo. Both groups received standard care, including hypoglycemic therapy and maximum tolerated dose of renin-angiotensin system (RAS) blockade therapy, such as angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptors Blocker (ARB).
The results showed that the study reached the primary endpoint: when combined with standard care, finerenone significantly reduced the risk of the composite primary endpoint of CKD progression, renal failure, and renal death compared with placebo. Specifically, with a median follow-up of 2.6 years, compared with placebo, finerenone will experience renal failure for the first time, the estimated glomerular filtration rate (eGFR) will continue to decrease from baseline by ≥40% for at least 4 weeks, and renal The compound risk of death was significantly reduced by 18% (HR=0.82; 95%CI: 0.73-0.93; p=0.0014). At 36 months, the number of treatments needed to prevent a primary composite endpoint event was 29 (95% CI: 16-166).
In addition, the study results showed that in the pre-specified subgroups, the effect of finerenone on the main results was generally consistent, and the treatment effect was sustained throughout the study period. With a median follow-up of 2.6 years, compared with placebo, finerenone also significantly reduced the risk of key secondary endpoints: a 14% reduction in the combined risk of cardiovascular death, non-fatal myocardial infarction, non-fatal stroke, or length of stay in hospital for heart failure ( The relative risk was reduced, HR=0.86 [95%CI: 0.75-0.99; p=0.0339]).
In this study, finerenone was well tolerated, consistent with the safety seen in previous studies. The overall adverse events and serious adverse events caused by the treatment were similar between the two groups. Most adverse events were mild or moderate. The frequency of serious adverse events in the finerenone group was lower than that in the placebo group (31.9% vs 34.3%). The incidence of hyperkalemia-related adverse events was higher (18.3% vs 9%). The two groups were severely related to hyperkalemia The incidence of adverse events was low (1.6% vs 0.4%), and there was no hyperkalemia-related death in the two groups. The proportion of patients who discontinued treatment due to hyperkalemia in the finerenone group was 2.0%, compared with 0.9% in the placebo group.
(source:internet, reference only)
Disclaimer of medicaltrend.org



