New drug for type 2 diabetes: Eli Lilly’s GIP/GLP-1 dual agonist tirzepatide
- Why Lecanemab’s Adoption Faces an Uphill Battle in US?
- Yogurt and High LDL Cholesterol: Can You Still Enjoy It?
- WHO Releases Global Influenza Vaccine Market Study in 2024
- HIV Infections Linked to Unlicensed Spa’s Vampire Facial Treatments
- A Single US$2.15-Million Injection to Block 90% of Cancer Cell Formation
- WIV: Prevention of New Disease X and Investigation of the Origin of COVID-19
Innovative new drug for type 2 diabetes: Eli Lilly’s GIP/GLP-1 dual agonist tirzepatide
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- What is the difference between Atorvastatin and Rosuvastatin?
- How long can the patient live after heart stent surgery?
New drug for type 2 diabetes: Eli Lilly’s GIP/GLP-1 dual agonist tirzepatide. Phase 3 tirals succeeded: reducing blood sugar, weight, and cardiovascular risk!
Eli Lilly recently announced the top-line results of a random, parallel, open-label, 52-week phase III clinical trial of SURPASS-4 (NCT03730662), a dual-effect GIP and GLP-1 receptor agonist, tirzepatide (LY3298176).
Data show that in adult patients with type 2 diabetes with increased cardiovascular (CV) risk, compared with titrated insulin glargine, all three doses of tirzepatide show superiority in reducing blood sugar and body weight: treatment for 52 weeks, with curative effect Estimation (efficacy estimand) statistical analysis method, the highest dose of tirzepatide (15 mg, once a week) reduces blood glucose level (A1C) from baseline by 2.58%, and weight from baseline by 11.7 kg (25.8 pounds, 13.0%), and titration Insulin glargine reduced A1C from baseline by 1.44% and body weight increased by 1.9 kg (4.2 lbs, 2.2%) from baseline.
In this patient population, the overall safety of tirzepatide is consistent with the class of glucagon-like peptide-1 (GLP-1) receptor agonists.
Gastrointestinal side effects are the most common adverse reactions, which usually occur during the dose escalation period and then decrease over time.
SURPASS-4 is the largest and longest trial in the SURPASS project so far, and it is also the fifth and final global registration study on tirzepatide for the treatment of type 2 diabetes.
‘The completion of the study was driven by the accumulation of Major Adverse Cardiovascular Events (MACE) to meet the regulatory submission requirements for assessing the cardiovascular risk of type 2 diabetes therapies.
The primary endpoint was measured at 52 weeks. Many patients continued to receive treatment after 52 weeks, and some patients continued for up to 2 years.
After a predetermined number of MACE events, a CV safety meta-analysis was conducted on the entire clinical project.
This meta-analysis included 116 patients who were judged to be MACE-4, which is the composite end point for cardiovascular or unexplained death, myocardial infarction, stroke, and hospitalization for unstable angina.
Compared with the pooled control drug, the risk ratio (HR) of pooled tirzepatide was 0.81 (97.85%CI: 0.52-1.26).
In the CV safety meta-analysis, the SURPASS-4 study contributed most of the MACE-4 events; in the SURPASS-4 study, the observed HR was 0.74 (95% CI: 0.51-1.08).
At present, the SURPASS project has met the regulatory submission requirements for assessing CV risks, and Eli Lilly intends to submit a complete registration data package to the regulatory authorities before the end of 2021.
Tirzepatide is a new type of glucose-dependent insulinotropic polypeptide (GIP, also known as gastric inhibitory polypeptide) receptor and glucagon-like peptide-1 (GLP-1) receptor dual activation developed by Eli Lilly.
Both GIP and GLP-1 are hormones secreted by the intestinal tract, which can promote insulin secretion.
Tirzepatide integrates the effects of two insulin-promoting effects into a single molecule and represents a new class of drugs for the treatment of type 2 diabetes.
The SURPASS Phase 3 global clinical development project has enrolled more than 13,000 type 2 diabetes patients in 10 clinical trials, 5 of which are global registration studies.
The project was launched at the end of 2018, and all five global registration trials have been completed.

Mike Mason, President of Eli Lilly’s Diabetes, said: “These strong results reinforce our belief that tirzepatide has the potential to be an exciting drug for patients with type 2 diabetes.
We look forward to achieving our goals for those suffering from this disease.
This disease group brings an important new treatment. We will share more detailed results at future scientific conferences and submit them to regulatory agencies later this year.”
John Doupis, MD, chief investigator of the SURPASS-4 trial and director of the Diabetes Department and Clinical Research Center of Iatriko Paleu-Falirou Medical Center in Athens, Greece, said: “In this study, tirzepatide has impressive effects compared to insulin glargine.
As a result, in type 2 diabetes patients with increased cardiovascular risk, it can significantly reduce the level of glycosylated hemoglobin (A1C) and significantly reduce weight.
Type 2 diabetes is a complex disease that requires individualized treatment methods, from SUPERSESS- 4 The results of the study show that for patients with type 2 diabetes, tirzepatide has the potential to become an important option that will help reduce blood sugar levels and weight.”
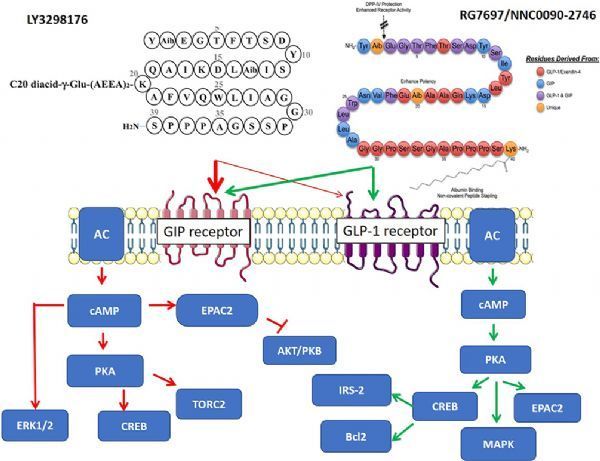
Tirzepatide (LY3298176, picture from literature: Dual GIP–GLP1-Receptor Agonists In The Treatment Of Type 2 Diabetes A Short Review On Emerging Data And Therapeutic Potential)
SURPASS-4 is a 52-week open-label global trial conducted in adult patients with type 2 diabetes with increased cardiovascular (CV) risk.
It evaluated 3 doses of tirzepatide (5mg, 10mg, 15mg) and titrated insulin glargine The efficacy and safety. The study enrolled more than 2,000 patients.
These patients have received 1-3 oral hypoglycemic drugs (metformin, sulfonylureas, SGLT2 inhibitors) but have poor blood sugar control. The average duration of diabetes is 11.8 years.
The baseline A1C was 8.52%, the baseline weight was 90.3 kg, and 85% of patients had a history of cardiovascular disease.
In the insulin glargine group, the insulin dose was titrated according to the treatment standard algorithm, with the goal of fasting blood glucose lower than 100 mg/dL.
The initial dose of insulin glargine is 10 units/day, and the average dose of insulin glargine at 52 weeks is 43 units/day.
In this study, two estimation methods (Efficacy estimate [Efficacy estimand] and treatment plan estimate [Treatment-regimen estimand]) were used to compare treatment differences.
Effectiveness estimand refers to the effect before stopping the study drug or starting rescue therapy to treat persistent severe hyperglycemia.
Treatment-regimen estimand refers to the efficacy of treatment of persistent severe hyperglycemia regardless of whether the study drug is adhered to or whether rescue therapy is used.
The results showed that the study reached the primary and key secondary endpoints using two measurement methods: all three doses (5mg, 10mg, 15mg) of tirzepatide had better blood glucose (A1C) and lower blood glucose (A1C) and key secondary endpoints than insulin glargine. The effect of reducing weight.
Among patients treated with the highest dose of tirzepatide (15 mg), 91% had A1C <7% (the treatment target for diabetic patients recommended by the American Diabetes Association [ADA]), and 43% had A1C <5.7% (this is in the absence of diabetes The level observed in the population). Specifically, the results at 52 weeks show:
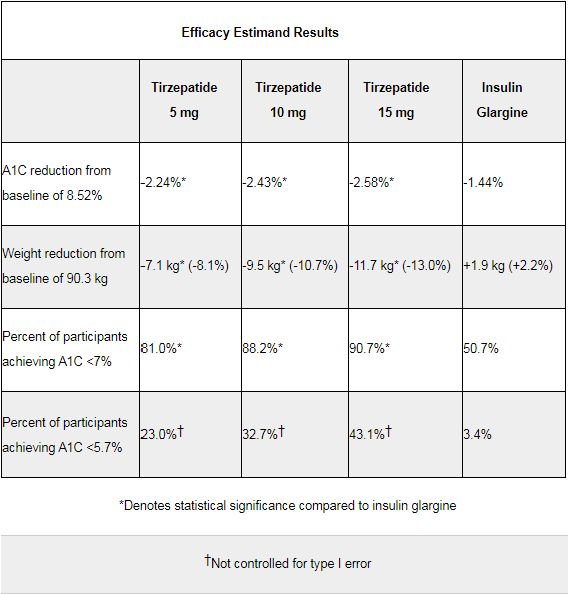
SURPASS-4 research data
In the estimation of the treatment plan, the three doses of tirzepatide were superior to insulin glargine in reducing A1C and body weight. The specific results are:
(1) A1C reduction: -2.11% (5mg), -2.30% (10mg), -2.41% (15mg), -1.39% (insulin glargine);
(2) Weight loss: -6.4 kg ( 5mg), -8.9kg (10mg), -10.6kg (15mg), +1.7kg (insulin glargine);
(3) The proportion of patients with A1C<7%: 75.1% (5mg), 82.9% (10mg), 84.9 % (15mg), 48.8% (insulin glargine).
(4) The proportion of patients with A1C<5.7%: 23.0% (5mg), 32.7% (10mg), 43.1% (15mg), 3.4% (insulin glargine).
(5) For hypoglycemia events <54 mg/dL during 52 weeks of treatment (Grade 2), the incidence of tirzepatide in each dose group was lower, which were 6.7% (5 mg), 5.5% (10 mg), and 6.5% (15 mg). , While the insulin glargine group was 15.0%.
In patients receiving background therapy with sulfonylurea drugs, episodes of hypoglycemia are more common.
During the 52-week treatment period, the most common adverse reactions were related to the gastrointestinal tract, generally mild to moderate.
Patients receiving tirzepatide (5mg, 10mg, 15mg) treatment, nausea (11.9% [5 mg], 15.9% [10 mg], 222.2% [15 mg]), diarrhea (12.2% [5 mg], 19.5% [10 mg], 20.4%[15 mg]), vomiting (4.9%[5 mg], 8.2%[10 mg], 8.3%[15 mg]) were more common than the insulin glargine group (1.6%[nausea], 3.2% [Diarrhea], 1.1% [vomiting]) higher.
During the 52-week treatment period, the discontinuation rates due to adverse events were 8.2% (5 mg), 7.3% (10 mg), 8.9% (15 mg), and 2.9% (insulin glargine).
Drinking alcohol can cause cancer, any alcohol has same Carcinogenicity
(source:internet, reference only)
Disclaimer of medicaltrend.org



