Mesenchymal stem cells: Effective in treating liver cirrhosis for a long time
- Popular Indian Spices Banned in Hong Kong Over Carcinogen Concerns
- AstraZeneca Admits for the First Time that its COVID Vaccine Has Blood Clot Side Effects
- Gut Bacteria Enzymes Offer Hope for ABO Universal Blood Transfusions
- Well-Known Japanese Medicine Exposed for 30 Years of Data Falsification
- Oregon Reverses Course: From Decriminalization to Recriminalization of Drug Possession
- Why Lecanemab’s Adoption Faces an Uphill Battle in US?
Mesenchymal stem cells: Effective in treating liver cirrhosis for a long time, improving liver function and health
- Israel new drug for COVID-19: EXO-CD24 can reduce deaths by 50%
- COVID-19 vaccines for children under 12 will be available soon
- Breakthrough infection of Delta: No difference from regular COVID-19 cases
- French research: ADE occurred in Delta variant and many doubts on it
- The viral load of Delta variant is 1260 times the original COVID-19 strain
Mesenchymal stem cells: Effective in treating liver cirrhosis for a long time. Latest results! Mesenchymal stem cells are effective in treating liver cirrhosis for a long time, improving liver function and increasing survival rate.
Recently, the latest research results of Chinese academician Wang Fusheng show that [1], umbilical cord mesenchymal stem cells significantly improve the liver function and long-term survival rate of decompensated cirrhosis. Relevant research results (preprints) are under consideration in Hepatology International.

A total of 219 patients were included in the study, of which 108 patients received stem cell therapy, 111 patients served as a control group, and were followed up for up to 75 months. The results showed that umbilical cord mesenchymal stem cells can reduce heparin transamination and significantly improve liver function, and the overall survival rate of the stem cell treatment group is higher than that of the control group. This study provides new evidence for the safety and effectiveness of mesenchymal stem cells in the treatment of liver cirrhosis.
End-stage liver disease and mesenchymal stem cell therapy
Alcohol, drugs and hepatitis viruses (HBV, HCV) can cause chronic liver damage, which can lead to liver cirrhosis and liver failure. Hepatitis B is highly prevalent in some countries. Every year, more than 9% of chronic hepatitis B patients progress to liver cirrhosis, and more than 5% of liver cirrhosis patients progress to liver cancer. Every year, 280,000 people die from hepatitis B related diseases. Countless hepatitis B patients are shrouded in the terrifying shadow of the hepatitis B-cirrhosis-liver cancer trilogy. End-stage liver diseases include cirrhosis and liver failure, with high mortality and heavy economic burden. Cirrhosis of the liver usually enters the decompensation phase irreversibly, which causes a series of complications, including ascites, esophageal-gastric varices bleeding, hepatorenal syndrome, hepatic encephalopathy, etc. [2].
Liver transplantation is the only option to improve the survival rate of patients with end-stage liver disease. However, there is a severe shortage of liver donors, high surgical costs, high surgical risks, potentially serious complications, and long-term use of immunosuppressive drugs after surgery. Therefore, gastroenterology/liver disease experts around the world have been searching for new and effective treatments for patients with end-stage liver disease with relatively small trauma and economic burden.
Mesenchymal stem cells are rich in sources and easy to obtain. They have the potential of multi-lineage differentiation and self-renewal ability. In addition, mesenchymal stem cells have low immunogenicity and are not easy to cause serious immune reactions.
During the treatment process, mesenchymal stem cells colonize the injured liver, and inhibit a series of complex mechanisms such as direct differentiation, paracrine or cell fusion, etc. The development of liver fibrosis promotes the reversal of liver cirrhosis. Therefore, mesenchymal stem cells have great potential in the treatment of end-stage liver disease.
Mesenchymal stem cells significantly improve the liver function of patients
According to the published literature [3], 513 patients who received stem cell infusion and met the criteria for liver failure and cirrhosis, after the infusion of mesenchymal stem cell therapy, the glutamate pyruvate aminotransferase (ALT) in the liver failure group and the cirrhosis group ), glutamate oxaloacetate aminotransferase (AST) and total bilirubin (TBIL) levels were significantly lower than baseline levels.

Four weeks after umbilical cord mesenchymal stem cell therapy, compared with patients who discontinued umbilical cord mesenchymal stem cell therapy, patients who received long-term treatment had a greater decline in TBIL levels.
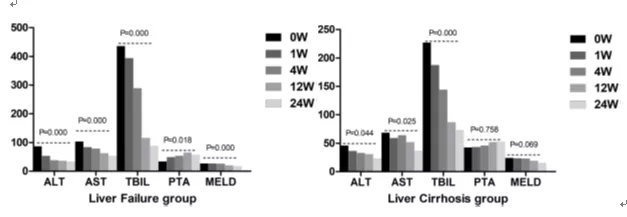
This means that the peripheral infusion of mesenchymal stem cells has a good therapeutic effect on liver failure and cirrhosis, and prolonging the course of treatment can improve the curative effect of mesenchymal stem cells on advanced liver disease.
Mesenchymal stem cells significantly reduce liver ascites
Another study [4] confirmed the safety and effectiveness of umbilical cord mesenchymal stem cell infusion in 30 patients with decompensated liver cirrhosis (LC) ascites, and compared it with 15 patients receiving saline infusion. Matched controls were compared.

Compared with the control group, mesenchymal stem cell therapy can significantly reduce ascites in patients with decompensated liver cirrhosis, increase serum albumin levels, and improve liver function. Serum albumin levels in the mesenchymal stem cell treatment group increased significantly at the 36th and 48th weeks (P <0.05). In the 4th week of mesenchymal stem cell treatment, serum cholinesterase levels increased significantly, which occurred earlier than the control group.
The study also performed ultrasound examinations on patients undergoing mesenchymal stem cell therapy, and found that the most prominent effect of mesenchymal stem cell therapy is that it can significantly reduce the amount of subgastric ascites and induce ascites remission, and the ascites in the mesenchymal stem cell therapy group disappeared The rate was significantly higher than that of the control group.
The related mechanism may be that mesenchymal stem cell therapy may play an anti-fibrosis effect and subsequently relieve portal hypertension. In addition, the activation of hepatic stellate cells is a key factor in the mechanism of liver cirrhosis, and mesenchymal stem cell therapy can reduce the activation of hepatic stellate cells through mesenchymal stem cell-derived IL-10 and tumor necrosis factor TNF-ɑ, or Induce apoptosis of hepatic stellate cells.
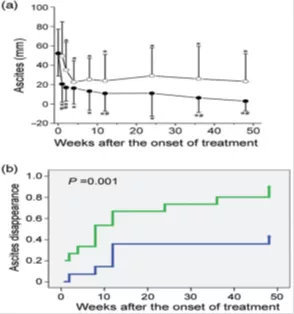
Compared with the control group, mesenchymal stem cell infusion improved thrombin function earlier in patients with decompensated liver cirrhosis and ascites. The platelet count continued to increase after 36 weeks of mesenchymal stem cell treatment. In the control group, they are only maintained at a low level. Therefore, at 36 and 48 weeks, the platelet count of patients treated with mesenchymal stem cells was significantly higher than that of the control group.
During the treatment, the study also monitored renal function parameters, such as urea, creatinine, and uric acid. In patients receiving mesenchymal stem cell therapy, serum creatinine levels were significantly reduced at the 8th week.
The safety of mesenchymal stem cell therapy is confirmed
Published studies showed that none of the patients reported short-term clinical adverse reactions, including pain, rash, infection, coma, or shock in the upper right abdomen. At the same time, by monitoring the incidence of complications throughout the trial, it was found that there was no statistical difference in the incidence of complications between the mesenchymal stem cell treatment group and the control group, including hepatocellular carcinoma, upper gastrointestinal hemorrhage, hepatic encephalopathy and pathogens. Peritonitis. These data confirm the safety of mesenchymal stem cell therapy.
All in all, more and more studies have confirmed the good therapeutic effect of mesenchymal stem cells in liver disease. It is believed that with the progress of clinical research, mesenchymal stem cell therapy will become a better choice for the majority of liver disease patients.
(sourceinternet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.