Cell Journal: Increasing the fidelity of protein synthesis can prolong lifespan
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Cell Journal: Increasing the fidelity of protein synthesis can prolong lifespan
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Cell Journal: Increasing the fidelity of protein synthesis can prolong lifespan
The culprit of aging has been found! Cell Journal: Increasing the fidelity of protein synthesis can prolong lifespan
At present, people’s researches on biological aging and diseases are endless, but most of them focus on DNA mutations, and there is much less research and understanding of the role of translation errors. In fact, protein translation errors are the most prone to errors in gene expression, and their frequency is thousands of times that of DNA mutations.
The imbalance of protein homeostasis is a key factor in aging and age-related diseases, and translation is one of its key determinants. Therefore, in the context of biological aging, it is very necessary to improve the understanding of the biological effects of translation errors. However, translation errors are rarely studied in the context of multicellular biological physiology. In addition, how to adjust the fidelity of protein synthesis to increase the lifespan of multicellular organisms is still a mystery.
On September 14, 2021, Ivana Bjedov, Filipe Cabreiro and other researchers from University College London, UK, jointly published an online publication in the journal Cell Metabolism entitled “
Increased fidelity of protein synthesis extends lifespan”. The article reveals that increasing the fidelity of protein synthesis can prolong life.

DOI: 10.1016/j.cmet.2021.08.017
The fidelity of protein translation is determined by the ribosomal decoding center. The RPS23 protein plays an important role in translation accuracy because it is indispensable in the process of domain closure and insertion of aminoacyl tRNA into the peptidyl transferase center. Therefore, the researchers used different databases to conduct an extensive unbiased phylogenetic analysis of RPS23 from archaea to eukaryotes, and found that the KQPNSA region of the RPS23 ribosome has a lysine residue that is very conserved, but lysine is present. The substitution of acid (K) to arginine (R). In order to study the link between this single point change and translation accuracy, the researchers used CRISPR/Cas9 to introduce a K60R mutation in the KQPNSA region of Drosophila RPS23.
The experimental results showed that the translation accuracy of RPS23 K60R fruit flies was improved compared with the control group. During the aging process of the control group, the errors of this site mutation increased significantly, but there was no change in the translation level between the wild type and the RPS23 K60R mutant. These findings indicate that translation intervention can improve accuracy without affecting global translation, and that in multicellular animals, the only ultra-precise mutation naturally selected by evolution will not damage the overall translation.
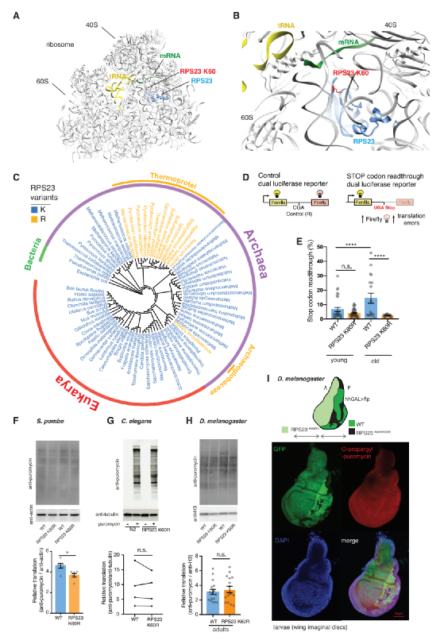
Mutations in RPS23 in the ribosome decoding center improve translation accuracy in Drosophila
Subsequently, the research team explored the physiological consequences of this mutation in yeast, worms and flies. Because the addition of the wrong amino acid, especially at the catalytic site of the protein, may cause harmful consequences, the error of the protein will increase the extra energy required for folding or protein degradation. In fact, the researchers observed in experiments that the RPS23 K60R mutation significantly increased the survival rate of yeast, worms, and flies under heat stress, reflecting their improved protein inhibitory ability, and the RPS23 K60R mutant was effective in yeast, worms, and worms. The flies exhibited developmental delay.
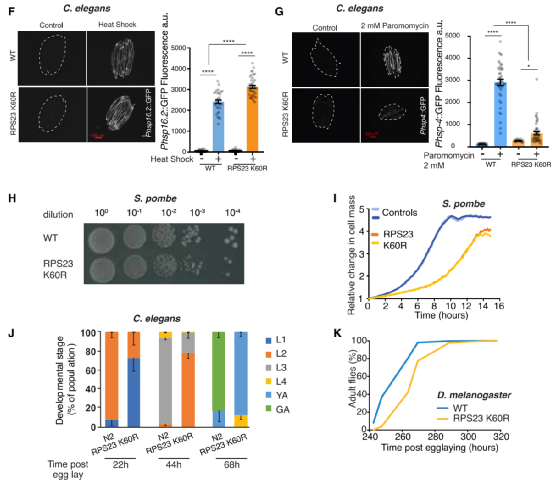 RPS23 K60R mutant exhibits heat tolerance and delayed development
RPS23 K60R mutant exhibits heat tolerance and delayed development
Finally, the researchers raised the question of whether improving the fidelity of translation can promote the longevity of unicellular and multicellular organisms. In repeated tests, it was found that the lifespan of the RPS23 K60R mutant was prolonged in yeast, worms and flies. This single-point mutation-mediated lifespan extension is 9%-23%. The use of negative geographic tropism or climbing test to measure the health of flies during aging shows that the increasing production capacity decreases as time goes by. However, compared with the control group, the RPS23 K60R flies climb The improvement shows that the mutant is healthier during aging.
In addition, the researchers showed that anti-aging drugs such as rapamycin, Torin1 and trametinib can reduce translation errors, and rapamycin can further extend the biological life of the RPS23 ultra-precision mutant. This means that different pharmacological anti-aging therapies have a unified mode of action.
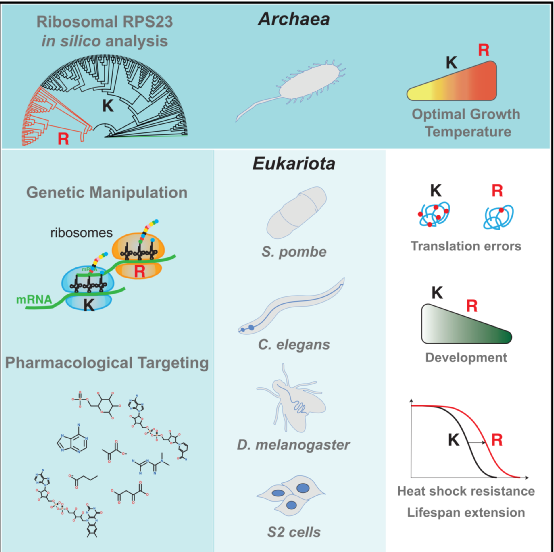 Experimental mechanism diagram
Experimental mechanism diagram
In summary, this article conducts a phylogenetic analysis of the key protein RPS23 in the ribosome decoding center, and introduces the eukaryotic RPS23 homologue. This mutation leads to accurate translation in yeast, worms and flies, as well as heat shock resistance. And a longer life. These findings paved the way for finding new translation accuracy interventions to improve aging.
Reference:
https://www.cell.com/cell-metabolism/fulltext/S1550-4131(21)00417-4
Cell Journal: Increasing the fidelity of protein synthesis can prolong lifespan
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



