Monkeypox mRNA Vaccine Competition: U.S. vs. China
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Monkeypox mRNA Vaccine Competition: U.S. vs. China
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Monkeypox mRNA Vaccine Competition: U.S. vs. China.
The first time scientists isolated monkeypox virus (Mpox virus, MPXV) from monkeys was in 1958 , and the first time people infected with monkeypox virus were found was in the 1970s .
Infected with monkeypox virus, you will have symptoms such as headache, fever, weakness, muscle pain, swollen lymph nodes, and rash.
It will last for about 2-4 weeks, and its fatality rate has recently been 3-6%.
For a long period of time, the human-to-human transmission of monkeypox virus was limited, and the recorded monkeypox virus infections were mainly concentrated in non-regions, and there has been no external expansion.

Nervously, since May 2022, monkeypox virus infection has continued to increase in both the scale and geographical extent of the epidemic.
By January 2023, the number of people infected with monkeypox had exceeded 8300 , including 75 deaths, in 110 countries.
In view of the fear of being dominated by the COVID-19 epidemic, the WHO took precautions and quickly listed the monkeypox epidemic as a public health emergency of international concern, so as to avoid people accusing it of being slow in responding to the crisis.
If you want to deal with the monkeypox epidemic that may get out of control, you have to get the vaccine in advance.
Speaking of which, the FDA has long approved two vaccines against monkeypox virus infection : one called JYNNEOS , an active non-replicating vaccine (MVAC) based on a modified vaccinia virus , was approved in 2019 ; one called ACAM2000 , which is a traditional live attenuated vaccine, was approved for marketing in 2007.
However, these two vaccines often cause some adverse reactions, and studies have found that the level of neutralizing antibodies against monkeypox virus triggered by JYNNEOS is very low.
Seeing the advantages and enthusiasm of the COVID-19 mRNA vaccine, people can’t wait to invest in the research and development of the monkeypox mRNA vaccine and grab the first apple.
We need to understand the monkeypox virus before we can talk about its vaccine development.
Monkeypox virus, the ancestor of the poxviridae orthopoxvirus genus, is its own brother with vaccinia, smallpox and rabbitpox, so if you are vaccinated against smallpox, it can also effectively prevent monkeypox virus infection, and the protection efficiency is not low.
To 85%, which suddenly makes the need to develop a monkeypox vaccine less urgent.
Monkeypox virus is an enveloped double-stranded DNA virus with a genome up to 200kb .
The novel coronavirus genome is about 29.9kb, the influenza virus genome is about 13.5kb, the HBV genome is about 3.2kb, and the HCV genome is about 9.6kb.
The monkeypox virus genome can encode at least 190 proteins, and the structures of more than 30 proteins have been solved.
Monkeypox virus has two infectious virion forms, one is the intracellular mature virion, called IMV , such as M1R, E8L, A29L, etc.; the other is the extracellular enveloped particle, called EEV , This EEV is based on the MV with an extra layer of envelope from the endoplasmic reticulum, on which some specific proteins are embedded, such as A35R, B6R and so on.
A27L, D8L, L1R, A33R and B5R in vaccinia virus have been identified as neutralizing antibody targets. It should be noted that the corresponding names of these proteins in monkeypox virus are: A29L, E8L, M1R, A35R and B6R.
There’s a lot of data showing that to trigger effective protection against poxviruses in animal models, you have to have a vaccine that contains multiple antigens from both IMV and EEV.
Take a look at the data. In the lethal vaccinia virus (VACV) challenge model, mice vaccinated with 5-valent protein subunit vaccines (L1R, D8L, A27L, A33R, and B5R) were more effective than mice vaccinated with 4-valent protein subunit vaccines (L1R ,, A27L, A33R and B5R) mice, the survival rate rose from 26% to 66%.
Let’s take a look, so far, who are developing monkeypox mRNA vaccines? How did they carry out antigen design? How about immunity?
Yale monkeypox mRNA vaccine
On November 29, 2022 , Sidi Chen’s team at Yale School of Medicine uploaded an article on BioRxiv : Polyvalent mRNA vaccination elicited potent immune response to monkeypox virus surface antigens .
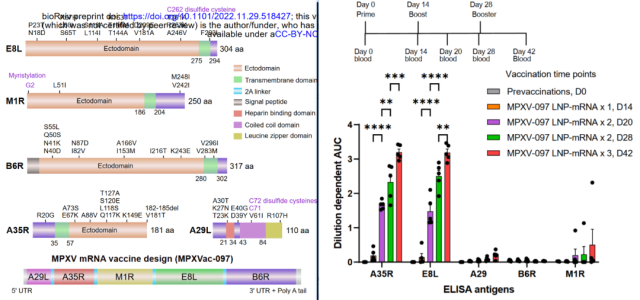
They designed a monkeypox mRNA vaccine encoding a multivalent antigen, called MPXVac-097 , which is composed of five neutralizing antibody target antigen fusions, namely A29L, E8L, M1R, A35R and B6R, and 2A self- Cleavage of peptides .
Immunization of mice with 3 doses of MPXVac-097 (8 μg) triggered significant A35R- and E8L-targeting-serum IgG, very low levels of M1R-targeting-serum IgG, and no detectable B6R- or A29-targeting-serum IgG.
Moderna monkeypox mRNA vaccine
On December 19, 2022 , the Moderna research team arrived late and uploaded an article on BioRxiv : A monkeypox mRNA-lipid nanoparticle vaccine targeting virus binding, entry, and transmission drives protection against lethal orthopoxviral challenge .
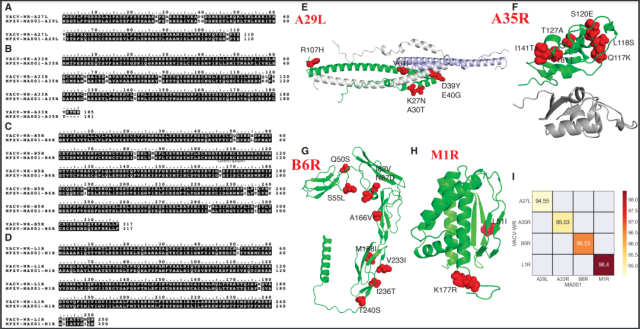
They selected 4 monkeypox virus antigens: A29L, M1R, B6R, A35R, and optimized the sequences of these 4 antigens, added signal peptides, reset glycosylation sites and modified transmembrane structures or cytoplasmic tails , to improve expression efficiency:
- A29L sequence : add the signal peptide of influenza virus H1 protein, remove all N-glycosylation sites and cysteine;
- B6R sequence : engineered sequence with truncated cytoplasmic tail (after residue 303) ;
- A35R sequence : an engineered sequence that adds the N-terminal transmembrane domain of the influenza virus N2 protein to replace the wild-type cytoplasmic and transmembrane regions (first 59 amino acids) ;
- M1R sequence : Add the signal peptide from influenza virus H1 to the M1 protein sequence, and mutate the threonine or serine codons in the M1 protein sequence to alanine codons, thereby removing all N-linked glycosylation sites point, also truncate the cytoplasmic region in the M1 protein sequence (after residue 208).
The neutralizing antibody titer of MPXV-targeted MPXV induced by single injection of M1-mRNA vaccine in mice was significantly higher than that of single injection of A29-mRNA vaccine, while single injection of B6 or A35 antigen could hardly induce targeting in mice. Neutralizing antibody to MPXV.
Interestingly, sera from mice immunized with B6-mRNA vaccine at only weak neutralizing antibody levels were able to block VACV transmission, whereas sera from mice immunized with M1-mRNA vaccine or MVA only weakly blocked VACV transmission .
In view of this, the researchers designed 2-valent/3-valent/4-valent monkeypox mRNA vaccines by combining antigens: M1+A35, M1+A35+B6, M1+A35+B6+A29 .
Compared with MVA, the 4-valent monkeypox vaccine was able to trigger significant serum-bound antibodies targeting the 4 antigens in mice, a strong Th1-biased humoral immunity, and a strong Fc receptor response.
In a lethal dose of VACV mouse infection model, both MVA and 2-valent/3-valent/4-valent monkeypox mRNA vaccines provided sufficient immune protection to prevent death of mice from infection.
SINOPHARM monkeypox mRNA vaccine
On November 21, 2022 , Yang Xiaoming’s team from SINOPHARM took the lead and announced the successful development of the world’s first monkeypox mRNA vaccine . An article was uploaded on BioRxiv : Novel mRNA vaccines encoding Monkeypox virus M1R and A35R protect mice from a lethal virus challenge .
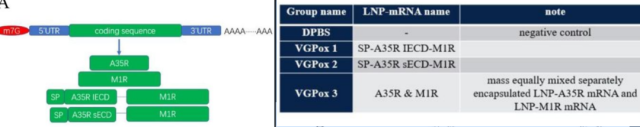
They chose M1R and A35R as antigens, and designed 3 mRNA vaccines: the first one is composed of fusion protein mRNA encoding full-length M1R + A35R with a complete extracellular domain (SP-A35R IECD-M1R) , called VGPox1 ;
The second type is composed of fusion protein mRNA encoding full-length M1R+ shortened extracellular domain A35R (SP-A35R sECD-M1R) , called VGPox2 ; the third type is composed of mRNA encoding A35R and M1R respectively, called VGPox3 .
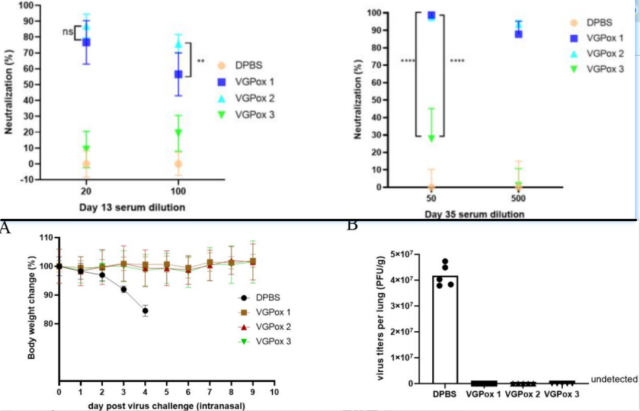
All three types of monkeypox mRNA vaccines (10μg) immunized mice could trigger anti-A35R serum IgGs.
In contrast, mice vaccinated with VGPox1/2 produced anti-M1R serum IgGs very early, while mice vaccinated with VGPox3 did not have detectable anti-M1R serum IgGs until 35 days later.
In addition, neutralizing antibodies and T cell immune responses also showed a similar phenomenon. Inoculation with three types of monkeypox mRNA vaccines could completely protect mice infected with lethal dose of VACV.
The mice in the immunized group had stable body weight and no symptoms, and the viral load in the lungs was 0. Taken together, VGPox1/2 has a better immune effect than VGPox3.
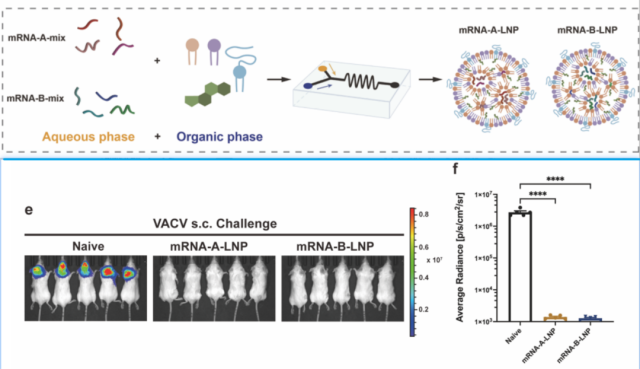
Monkeypox mRNA vaccine by Wang Shengqi’s team at Military Medical College
On November 23, 2022 , one step too late, and missed the title of developing the world’s first monkeypox mRNA vaccine, Wang Shengqi’s team from the Military Medical College uploaded an article on BioRxiv : Monkeypox virus quadrivalent mRNA vaccine induces antibody responses and cellular immunity and protects mice against Vaccinia virus .
They constructed 4 kinds of mRNA sequences, respectively encoding 4 kinds of monkeypox antigens: A29, M1R, A35R, B6R .
Four kinds of mRNA sequences were encapsulated together by LNP to construct a 4-valent mRNA monkeypox vaccine .
After immunization of mice, this 4-valent monkeypox mRNA vaccine (40 μg) triggered significant serum IgG antibodies targeting MPXV antigens, neutralizing antibodies targeting VACV and long-lasting MPXV-specific memory T cells and B Cellular response, effectively protecting VACV-challenged mice.
Qin Chengfeng Monkeypox mRNA Vaccine, Military Medical College
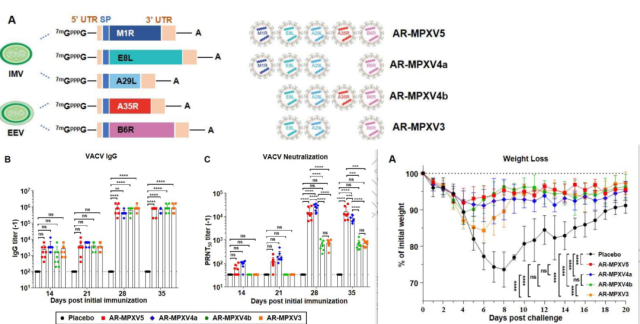
On March 22, 2023 , Qin Chengfeng’s team from the Military Medical College published an article in Emerging Microbes & Infection : Rational development of multicomponent mRNA vaccine candidates against mpox .
They designed 5 kinds of mRNA sequences, respectively encoding 5 kinds of monkeypox antigens: M1R, E8L, A29L, A35R, B6R , combined to construct 4 types of multivalent monkeypox mRNA vaccines.
Four types of multivalent monkeypox mRNA vaccines immunized mice (containing 5 μg of mRNA encoding each antigen) , all of which could strongly trigger serum IgG targeting VACV, neutralizing antibodies targeting VACV, and MPXV-specific Th1 bias cellular immunity.
All four types of mRNA vaccines prevented weight loss in mice infected with high-dose VACV.
After 8 days of VACV infection, viral DNA or infectious virus particles were basically undetectable in the lungs and upper respiratory tract.
It is worth noting that the 5-valent vaccine AR-MPXV5 and the 4-valent vaccine AR-MPXV4a exhibited stronger induction of neutralizing antibodies and better immune protection.
Sum up:
In the antigen selection of monkeypox mRNA vaccine, it must contain surface antigens from both IMV and EEV .
The most optimized and modified antigen sequence must be the R&D team of Moderna.
Antibody titers and cellular immunity triggered by different antigen combination forms and different expression patterns were different, but all types of combinations could protect mice infected with lethal dose of MAVC to survive.
Everyone chooses the MAVC-infected mouse model to evaluate the protective effect of monkeypox mRNA vaccine. It may be that the MPXV-infected mouse model is not easy to establish.
In comparison, everyone is still more willing to adopt the method of LNP encapsulation of multiple mRNA vaccine sequences to construct a multivalent vaccine, which is simple and highly operable.
If you fuse multiple antigens into one protein expression, the requirements for sequence design are high, and it is difficult to control, and it is difficult to ensure that the immunogenicity of the translated protein is not damaged.
The existing research and development results of monkeypox mRNA vaccine have at least accumulated experience for the construction of multivalent mRNA vaccine, and no one should be willing to do it later.
Monkeypox mRNA Vaccine Competition: U.S. vs. China.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



