This review creatively proposes eight hallmarks of cardiovascular aging
- Aspirin: Study Finds Greater Benefits for These Colorectal Cancer Patients
- Cancer Can Occur Without Genetic Mutations?
- Statins Lower Blood Lipids: How Long is a Course?
- Warning: Smartwatch Blood Sugar Measurement Deemed Dangerous
- Mifepristone: A Safe and Effective Abortion Option Amidst Controversy
- Asbestos Detected in Buildings Damaged in Ukraine: Analyzed by Japanese Company
This review creatively proposes eight hallmarks of cardiovascular aging
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
This review creatively proposes eight hallmarks of cardiovascular aging.
Normal circulatory function is a key determinant of disease-free life expectancy (healthspan) .
In fact, diseases affecting the cardiovascular system are becoming more prevalent and a leading cause of morbidity, disability and death worldwide, making maintaining cardiovascular health essential to promoting good health and longevity.
On May 16, 2023, Prof. Guido Kroemer from the CARPEM Cancer Institute in Paris, France, and Prof. Mahmoud Abdellatif from the Department of Cardiology, Graz Medical University, Austria, published a paper entitled: Hallmarks of cardiovascular ageing in Nature Reviews Cardiology , a review journal under Nature.
This review creatively proposes eight hallmarks of cardiovascular aging — loss of macrophage function , loss of protein homeostasis , genome instability , epigenetic alterations , mitochondrial dysfunction , cellular senescence , neurohormonal signaling dysregulation , and inflammation . It also discusses how these features can be used to mitigate cardiovascular risk.

This review creatively proposes eight hallmarks of cardiovascular aging.
The human cardiovascular system is composed of many different cell types, including endothelial cells, smooth muscle cells, cardiomyocytes, fibroblasts, and immune cells, that work in concert to maintain an adequate blood supply and waste disposal to every cell in the body.
Notably, most cardiovascular cell types are highly specialized and need to function under harsh conditions characterized by constant mechanical and shear stress. However, the acquisition of unique functions (such as cardiomyocytes, the only contractile cell type in the heart) comes at the expense of the cell cycle.
Thus, these terminally differentiated cells are densely packed with mitochondria and sarcomeres. Aging inevitably erodes the physiological function of every cell type in the body, and cardiomyocytes and other cardiovascular cells are also particularly prone to age-related dysfunction and failure.
Indeed, the heart, blood vessels, and microcirculation undergo substantial structural and functional remodeling with age. So it’s no surprise that age is one of the strongest predictors of cardiovascular health.
Furthermore, despite remarkable advances in the medical management of cardiovascular risk factors, pathologies affecting the cardiovascular system remain a major cause of morbidity, disability, and mortality worldwide.
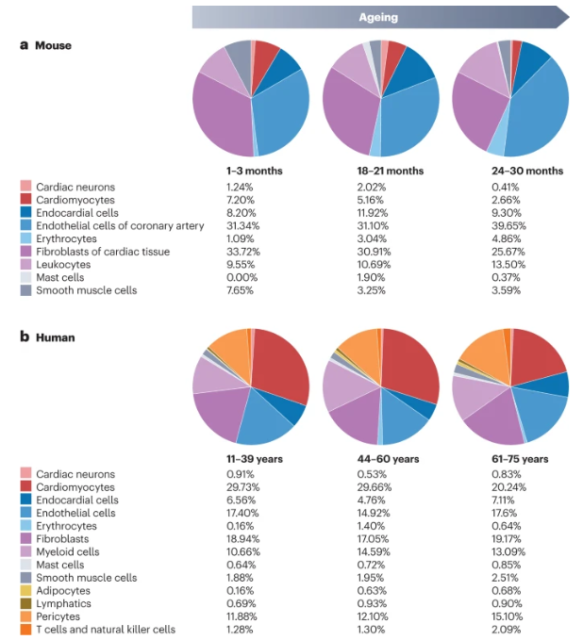 Effects of aging on the cellular composition of the cardiovascular system
Effects of aging on the cellular composition of the cardiovascular system
In this review, the researchers speculate that persistently high rates of cardiovascular disease and mortality are due to a lack of effective interventions targeting the aging process itself. Interestingly, experimental interventions specifically designed to delay cardiovascular aging are sufficient to attenuate aging in model organisms.
Similarly, targeting vascular endothelial growth factor (VEGF) signaling prevented age-related microvascular wear and effectively delayed age-related pathology across multiple organ systems, extending lifespan in mice.
These examples confirm the central role of cardiovascular deterioration in mediating systemic aging and suggest that targeting the circulatory system is a viable entry point to promote healthy aging at the organismal level.
The premise of anti-aging interventions is to target the molecular mechanisms of aging. However, many classic “signatures of aging” were originally discovered in invertebrate model organisms.
Furthermore, although these hallmarks were revised in 2023 to focus on aging in mammals, their specific involvement in the dysfunction of major cardiovascular cell types during aging remains to be assessed.
In this review, eight molecular markers are proposed as common features of cardiovascular system aging based on three stringent criteria:
1. The phenomenon must manifest itself in the aging heart, blood vessels, or both.
2. Genetic manipulations that promote markers must accelerate signs of cardiovascular aging.
3. Experimental or therapeutic inhibition of this marker could delay, halt or reverse cardiovascular aging.
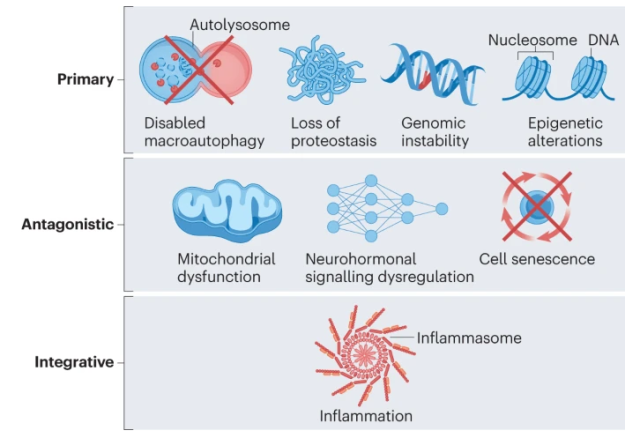 Cardiovascular Aging Hallmarks
Cardiovascular Aging Hallmarks
These hallmarks include major mechanisms of aging, namely loss of macrophage function , loss of proteostasis , genomic instability , epigenetic alterations , mitochondrial dysfunction , cellular senescence , dysregulation of neurohormonal signaling , and inflammation , which we address below.
1. Loss of macrophage function
Macroautophagy, a key cellular quality control mechanism, is essential for the clearance of dysfunctional cytoplasmic components .
During autophagy, potentially toxic protein aggregates and aged organelles are sequestered in double-membrane vesicles, and autophagosomes subsequently fuse with lysosomes to be enzymatically degraded, thereby providing metabolism for bioenergetic and anabolic reactions things.
In addition, the autophagic machinery contributes to “heterophagy”, in which cardiomyocytes package dysfunctional mitochondria in the so-called exosphere, extruding the waste into the extracellular space, where it is degraded by heart-resident macrophages .
Given its important homeostatic role, autophagy is widely accepted to control the healthspan and lifespan of various eukaryotic cell types, especially those that make up the cardiovascular system.
In fact, dysfunctional autophagy has been linked to a wide range of cardiovascular diseases, including atherosclerosis, coronary heart disease, diabetic cardiomyopathy, chemotherapy-induced cardiotoxicity, cardiac arrhythmias, and heart failure.
In addition, several widely used cardioprotective drugs, including aspirin, β-adrenergic blockers, and calcium channel blockers, have been shown to modulate autophagic activity.
Accumulating evidence indicates that autophagy function declines with aging, which contributes to the pathogenesis of age-related chronic diseases.
In the cardiovascular system, autophagy has been reported to gradually decrease in the vasculature of aged mice and humans.
In contrast, whether and how aging affects cardiac autophagy has been a matter of debate. Thus, improved methods for assessing autophagic flux have been used in studies in Drosophila, mice, and rats, providing irrefutable evidence of decreased autophagy in the aging heart as well as in the aging vasculature.
Multiple mechanisms are involved in the age-related decline in autophagy. Among these mechanisms, inhibitory hyperacetylation of autophagy proteins is associated with reduced activity of sirtuin deacetylase and its essential substrate NAD+ in the aged cardiovascular system.
Inhibition of autophagy may also be due to increased nutrient availability, which provides excess acetyl-CoA levels for protein acetylation, and hyperactivation of the insulin-IGF1-serine/threonine protein kinase mTOR signaling pathway, especially Obesity and metabolic syndrome in the presence of (visceral) .
Furthermore, age-related decreases in the levels of the polyamine spermidine may impair autophagy-dependent translation of proteins. Indeed, cardiac spermidine content gradually declines with age in humans, and its exogenous supplementation reactivated cardiac and vascular autophagy in aged mice.
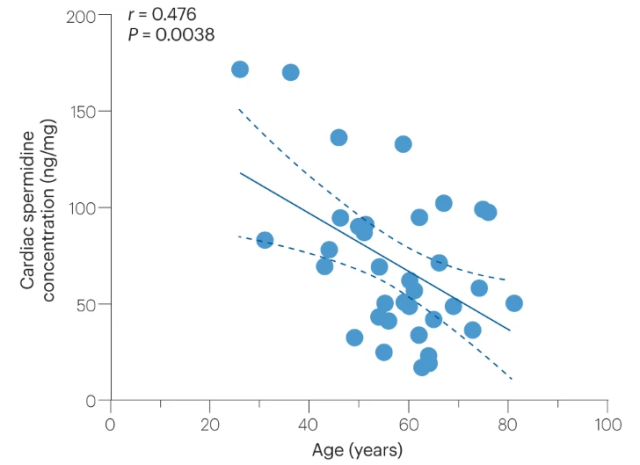 Age-dependent decline in human cardiac spermidine levels
Age-dependent decline in human cardiac spermidine levels
2. Loss of protein homeostasis
Protein homeostasis is a major driver of organismal health and resilience, with the delicate balance between protein synthesis, folding, and degradation tightly regulated by an extensive network of molecular chaperones and proteolytic enzymes . The integrity of the proteome is maintained by refolding denatured proteins or their selective degradation.
Age-related loss of proteostasis poses a particular challenge to cardiomyocytes because their functional unit, the sarcomere, relies on proteostasis for maintenance. Cardiomyocytes, however, face an abundance of protein-toxic reactive oxygen species that are byproducts of intense oxidative phosphorylation.
Aged rat hearts have been shown to accumulate oxidized and ubiquitinated proteins, which correlate with reduced proteasome activity and expression levels of several HSPs. Notably, vascular mediator aggregates are associated with cerebrovascular dysfunction in aged mice and cognitive decline in patients with vascular dementia or Alzheimer’s disease.
The causal role of protein instability in cardiovascular aging is supported by experimental studies in which cardiovascular aging is exacerbated (or delayed) by inducing a loss of proteostasis in the cardiovascular system .
Taken together, the data suggest that age-related cardiovascular decline results in a loss of proteostasis and suggest that genetic, dietary, or pharmacological strategies to maintain proteostasis can delay cardiovascular aging.
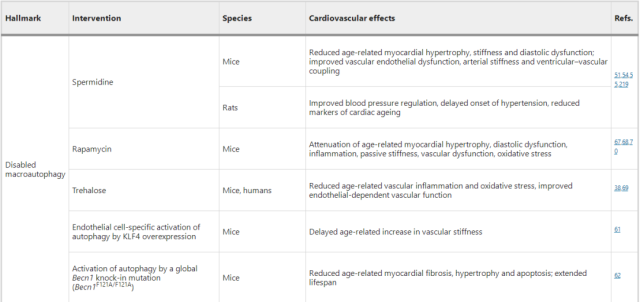 Interventions to Extend Cardiovascular Healthyspan, Partially by Targeting Signatures of Aging
Interventions to Extend Cardiovascular Healthyspan, Partially by Targeting Signatures of Aging
3. Genome instability
Although genomic instability is a common feature of aging in various organs and across species, the rate of mutation accumulation varies widely between tissues. In the cardiovascular system, age-associated mutations appear to be strongly tissue and context dependent.
The relationship between DNA stability and vascular aging is less controversial, although aging appears to induce greater DNA damage and telomere dysfunction in vascular endothelial cells compared with smooth muscle cells .
DNA damage has also been reported in human atherosclerotic plaques, and mice with vascular smooth muscle cell-restricted DNA damage exhibit lower atherosclerotic plaque stability.
In addition, comprehensive and systematic genomic analyzes assessing vascular DNA damage in naturally aging mice and humans over the course of life are needed to confirm the relevance of these findings.
These studies will also help to pinpoint the precise role of genomic instability in vascular aging, as genetic models of radiation-induced forced DNA damage reveal systemic abnormalities and other mechanisms that may contribute to vascular dysfunction, regardless of DNA integrity status.
4. Epigenetic changes
Independent of DNA mutations, gene expression levels in the cardiovascular system may be affected by a variety of epigenetic factors, including DNA methylation, histone modifications, and noncoding RNAs (ncRNAs) .
These epigenetic alterations focus on age-related changes and gene transcriptional imbalances, implicated in pathophysiological processes such as loss of protein homeostasis, mitochondrial dysfunction, and inflammation.
Even in the heart, where genomic instability is disputed, aging leads to marked heterogeneity in gene expression, suggesting that epigenetic alterations may be involved in cardiovascular aging.
5. Mitochondrial dysfunction
Mitochondrial dysfunction is a key feature of aging and age-related chronic diseases, especially in the cardiovascular system, which is heavily dependent on mitochondrial metabolism and signaling.
In addition to their function in cardiac bioenergetics, mitochondria also play a role in intracellular calcium homeostasis, redox balance, anabolic and catabolic responses, and the initiation of inflammatory and lethal signals in virtually all cell types in the cardiovascular system.
Crucial role. Thus, age-related mitochondrial dysfunction first affects the cardiovascular system. In fact, mitochondrial dysfunction is arguably one of the most critical etiologies in a wide range of cardiovascular diseases.
In the heart, aging is associated with a dramatic decline in mitochondrial content . Furthermore, aging cardiac mitochondria are structurally and functionally compromised, manifested by increased diameter (swelling) , matrix deformation, loss of cristae, and oxidation secondary to reduced activity and stability of mitochondrial respiratory chain complexes (especially complex IV) Phosphorylation decreased.
Importantly, interventions aimed at avoiding some aspects of age-related mitochondrial dysfunction have been shown to be cardiovascular protective and delay aging.
For example, mitophagy induced in cardiomyocytes by transgenic overexpression of the E3 ubiquitin protein ligase parkin not only maintained mitochondrial integrity and cardiac function, but also attenuated signs of aging and inflammation in aged mice.
Altogether, these findings suggest that mitochondria are a viable target for preventing or delaying cardiovascular aging at the preclinical level, and attempts to translate these findings into the clinic are needed.
6. Cell aging
Senescence is generally defined as a permanent arrest of the cell cycle, resulting in irreversible loss of replicative capacity as well as reduction of specific cellular functions and acquisition of proinflammatory features .
However, senescence does not only apply to proliferating cell types, such as endothelial cells and fibroblasts, but can also affect cardiomyocytes.
Aging is induced by a variety of internal and external stressors, including telomere shortening, oncogenic signaling, persistent DNA damage, oxidative and mechanical stress, nutritional imbalances and mitochondrial dysfunction, and viral or bacterial infections.
Due to their anatomical location, vascular endothelial cells , including cells in the microcirculation of the brain, are directly exposed to senescence-inducing stimuli, including biochemical and hemodynamic factors.
Senescent endothelial cells exhibit abnormal morphology and enlargement, as well as increased adhesion to the basement membrane and reduced ability to align with the direction of blood flow, compared with their normal counterparts.
Functionally, senescent endothelial cells are associated with age-related endothelium-dependent vasodilation dysfunction and loss of blood-brain barrier integrity.
However, in naturally aging mice, systemic clearance of senescent cells delayed aging and extended lifespan .
In the cardiovascular system of these animals, genetic or pharmacological elimination of senescent cells attenuated cardiac hypertrophy and fibrosis and preserved cardiac function.
Taken together, these findings suggest that accumulation of secretory and metabolically active senescent cells in the cardiovascular system may affect various aspects of cardiovascular aging.
Therefore, targeting senescent cells may be a promising approach to delay age-dependent decline in cardiovascular fitness.
7. Dysregulation of neurohormonal signaling
Major components of systemic and local neurohormonal signaling, including the renin-angiotensin-aldosterone system (RAAS) , β-adrenergic signaling, and insulin-IGF1 signaling, are chronically activated during aging, leading to cardiac Disorders of the vascular system.
8. Inflammation
Aging is associated with chronic low-grade sterile inflammation, or inflammation, which is particularly evident in age-related cardiovascular diseases such as atherosclerosis.
In fact, atherosclerosis is a decades-long inflammatory lesion involving multiple immune cell subtypes and cytokines, combined with risk factors such as dyslipidemia, leading to vascular remodeling and plaque formation and rupture.
More strikingly, a T-cell-specific defect in mitochondrial transcription factor A promotes chronic inflammation and accelerates aging, including in the cardiovascular system, which can be attenuated by TNF neutralization. Thus, immune senescence affecting T lymphocytes can accelerate cardiovascular aging.
In addition to atherosclerosis and associated coronary artery disease, HFpEF (the predominant form of heart failure in the elderly) is also recognized as an inflammatory multiorgan syndrome primarily driven by accelerated aging.
In aged mice fed a high-fat diet and deoxycorticosterone, the development of HFpEF could be prevented by administration of β-hydroxybutyrate, which acts directly on the NLPR3 inflammasome, inhibiting IL-1β maturation and Pro-inflammatory, cytokine-induced mitochondrial dysfunction.
As mentioned earlier, there is substantial evidence supporting the idea that inflammatory processes contribute to cardiac and vascular aging.
However, which patient populations would benefit from anti-inflammatory interventions and whether multiple cell types (e.g., macrophages and T lymphocytes) , intracellular pathways (e.g., cGAS–STING, NF-κB, and inflammasome pathways) were simultaneously targeted Or pro-inflammatory effector molecules (such as type I interferon, TNF and IL-1β) may constitute an effective combination strategy to delay or prevent cardiovascular aging remains to be determined.
In conclusion (This review creatively proposes eight hallmarks of cardiovascular aging)
Collectively, the hallmarks of cardiovascular aging are tightly intertwined, meaning that experimental exacerbation or attenuation of any single marker affects most of the others.
For example, disrupted autophagy leads to loss of protein homeostasis, mitochondrial dysfunction, dysregulation of neurohormonal signaling, and increased inflammation.
Conversely, experimental induction of autophagy also improved mitochondrial function, prevented loss of protein homeostasis, reduced cellular senescence and attenuated inflammation.
This article presents a didactic distinction between hallmarks of cardiovascular aging, namely the first four hallmarks discussed in this review (loss of macroautophagy function, loss of proteostasis, genomic instability, and epigenetic alterations) , which increase with age.
The gradual development of the growth and composition of the genome, epigenome, proteome and organelles should be considered as the main marker of cardiovascular aging initiated at the upstream level.
Further downstream, it is hypothesized that mitochondrial dysfunction, dysregulation of neurohormonal signaling and cellular senescence are antagonistic markers reflecting (mal) adaptive responses to injury and thus have a more subtle role in cardiovascular aging.
Of note, continued exposure to external factors, such as malnutrition, sedentary lifestyles, psychological and social stress, smoking and pollution, may also contribute to the development of cardiovascular disease by acting on these markers at different levels .
This concept is exemplified by the major cardiovascular risk factors of obesity, where loss of autophagy, loss of proteostasis, epigenetic alterations, mitochondrial dysfunction, cellular senescence, dysregulation of neurohormonal signaling, and chronic inflammation have been reported and are associated with The accelerated cardiovascular aging phenotype is consistent.
In contrast, modest weight loss induced by dietary restriction has shown promising cardiovascular and other antiaging benefits, including in nonobese mice and humans.
Virtually every marker of cardiovascular aging can be considered as a potential target for the development of new drugs to extend cardiovascular healthy lifespan.
Theoretically, the degree to which these markers are interconnected will determine whether drugs targeting different features of cardiovascular aging can be advantageously combined to produce additive, and possibly even synergistic, effects.
Systematic evaluations of simultaneous interventions in different aspects of the aging process, as well as outcome measures in cardiovascular and general health, are therefore warranted.
Despite extensive preclinical evidence, more research is needed to solidify the translational relevance and targeting of aging signatures in humans. Although animal models mimic many aspects of human aging, they cannot fully capture its complexities.
Beyond pharmacotherapy, an in-depth understanding of the extent to which lifestyle interventions such as diet and exercise can be optimized to adapt the cardiovascular system to various environmental stressors and slow the aging process is needed.
Indeed, there is a growing literature on the molecular basis of healthy diet, regular physical activity, and appropriate psychosocial stress responses.
Therefore, in future research, our goal should be to establish objective, biomarker-guided recommendations for incorporating specific pharmacological prevention strategies to develop healthy habits.
Paper link:
https://www.nature.com/articles/s41569-023-00881-3
This review creatively proposes eight hallmarks of cardiovascular aging
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



