Co-express gag protein and chimeric Spike to develop VLP COVID-19 mRNA vaccine
- A Single US$2.15-Million Injection to Block 90% of Cancer Cell Formation
- WIV: Prevention of New Disease X and Investigation of the Origin of COVID-19
- Why Botulinum Toxin Reigns as One of the Deadliest Poisons?
- FDA Approves Pfizer’s One-Time Gene Therapy for Hemophilia B: $3.5 Million per Dose
- Aspirin: Study Finds Greater Benefits for These Colorectal Cancer Patients
- Cancer Can Occur Without Genetic Mutations?
Co-express gag protein and chimeric Spike to develop VLP COVID-19 mRNA vaccine
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
PNAS | Co-express gag protein and chimeric Spike to develop VLP COVID-19 mRNA vaccine.
The envelope glycoprotein (Envelope glycoprotein, Env) trimer on the surface of HIV-1 virus is responsible for recognizing host cell surface receptors and completing the membrane fusion process, and is the only target recognized by neutralizing antibodies.
HIV-1 Env is a trimer formed by heterodimer monomers, each monomer contains receptor binding protein gp120 (Surface glycoprotein, SU) and transmembrane fusion protein gp41 (Transmembrane protein, TM) , to Linked by non-covalent interactions.
The precursor Env protein (gp160) is synthesized in the endoplasmic reticulum and transported to the Golgi apparatus for related glycosyl modification and protein folding to form a trimer conformation, and then gp160 is cleaved into gp120 by the Furin family and gp41 subunits. gp41 is anchored to the viral envelope through the transmembrane region, and the C-terminal cytoplasmic domain is located in the viral lumen.
Gag protein is the precursor structural protein of HIV-1 virus, which can be hydrolyzed by protease to produce matrix protein (MA) , coat protein (CA) , nucleocapsid protein (NC) , p6 and so on.
Matrix proteins are inserted into the cytoplasmic bilayer of the viral envelope, while capsid proteins, nucleocapsid proteins, and viral RNA condense to form the viral core.
The gag protein is able to self-assemble into virus-like particles (VLPs) regardless of the presence of other viral components. The CTD of the gp41 protein interacts with the matrix domain (MA) of the gag protein and plays an important role in HIV-1 viral budding and release.
In addition, the core antigen and various enzyme proteins of HIV are relatively conservative, even among HIV-1, HIV-2 and SIV, they also have high homology, and the variation is mainly from the envelope protein.
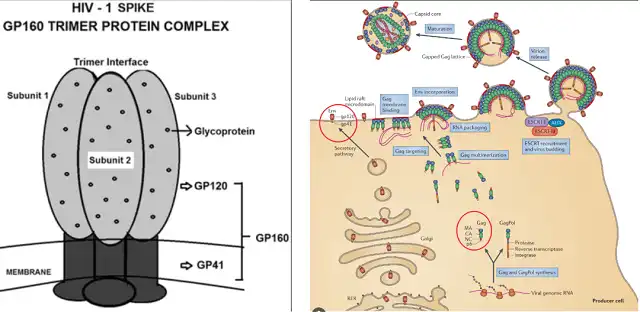
HIV-1 Spike Protein Complex
Left image source : Home Global Virology II – HIV and NeuroAIDS ChapterHIV-1 Envelope (ENV) GP160 Trimer Protein Complex SPIKE as a Recombinant Macromolecular Assembly Vaccine Component Candidate: Current Opinion
Right image source : HIV-1 assembly, release and maturation.
In 1986 , Professor Paolo Lusso came to NIH for the first time and worked in the Tumor Cell Biology Laboratory of Dr. Robert C. Gallo of the National Cancer Institute.
In 1994 he returned to Italy to create the Human Virology Laboratory at the San Raffaele Institute of Science in Milan and became Associate Professor of Infectious Diseases at the University of Cagliari.
In 2006, he joined NIH again as the Viral Pathogenesis Group of the Immunomodulation Laboratory, and has long been committed to the research and development of HIV vaccines.
In 2021, Paolo Lusso et al. developed an HIV-1 mRNA vaccine that can encode and assemble VLPs .
In the same cell, HIV-1 Env protein and SIV (Simian Immunodeficiency Virus) gag protein are co-expressed for efficient assembly and Release VLPs .
In vivo data in mice showed that VLP-based env-gag mRNA vaccines elicited stronger neutralizing antibodies than env mRNA vaccines alone.
Based on this, on July 10, 2023 , Paolo Lusso’s team published an article in PNAS : Increased neutralization potency and breadth elicited by a SARS-CoV-2 mRNA vaccine forming virus-like particles , they applied the same VLP design strategy to The COVID-19 mRNA vaccine hopes that through optimization, this VLP COVID-19 mRNA vaccine can trigger an immune response more efficiently than the conventional Spike-encoded COVID-19 mRNA vaccine, and induce more extensive and durable neutralizing antibodies.
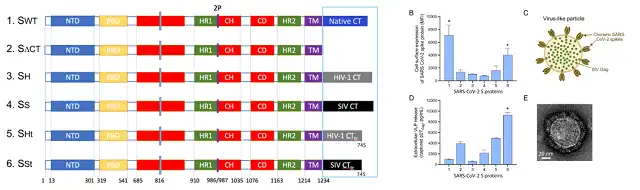
Design chimeric antigens to form VLPs
First, Paolo Lusso selected Spike protein as the vaccine antigen, and added 2P (K986 and V987) mutation sites, and at the same time, removed the Furin cleavage site.
Considering that the gag protein interacts with the gp41 protein CTD to promote HIV-1 or SIV virion assembly and release , they designed chimeric Spike antigens by replacing the Spike protein CTD with the gp41 protein CTD sequence from HIV-1 or SIV sequence.
Transfect the cells with various designed mRNAs, and detect the expression level of Spike protein on the cell surface.
The mRNA encoding native Spike was expressed at the highest level on the cell surface, and once the native CTD region was removed, the expression on the Spike cell surface dropped sharply, indicating that the CTD region is very important for the process of Spike protein transport to the cell membrane .
Among the chimeric Spike proteins, only the Spike protein (Sst) connected to the SIV truncated CTD had a higher cell surface expression level , and the other chimeric Spike protein cell surface expression levels were all low.
The mRNA encoding Spike protein and the mRNA encoding SIV gag protein were simultaneously transfected into cells at a mass ratio of 1:2, and the VLP secreted by the cells was detected. Although native Spike mRNA expression was the highest, secreted VLPs were extremely low, whereas CTD truncated Spikes secreted higher VLPs.
For chimeric Spike proteins, VLP secretion was highest for Spike (Sst) linked to the SIV truncated CTD.
Immunogenicity Comparison
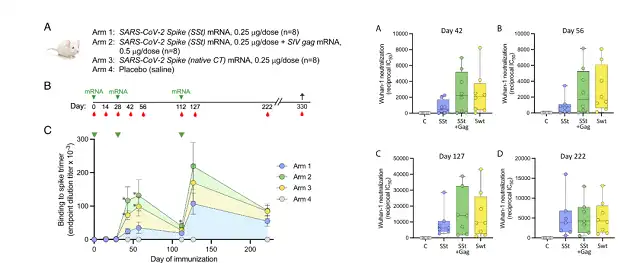
The researchers designed 4 groups of mouse immunization schemes, using intramuscular injection, and the immunization time was 0/4/16 weeks:
- The first one, inject 0.25ug Sst mRNA-LNP ;
- The second one, inject 0.25ug Sst mRNA-LNP+0.5ug gag mRNA-LNP ;
- The third one, inject 0.25ug Spike mRNA ( natural CTD )
- Fourth, inject saline.
From the first injection to the 330th day of immunization, the highest antibody titer in the serum of mice was Sst+gag mRNA , which was significantly higher than that of mice inoculated with Spike mRNA (natural CTD) or only inoculated with Sst mRNA.
In addition, we can see that when the third injection is given, that is, on day 112, the serum binding antibody titer has decayed to the lowest level, but 5 days after the third injection, the serum binding antibody titer will soar again, and then As time goes on, it starts to decay again.
From the first dose of immunization to the 330th day of immunization, for neutralizing antibodies targeting the original strain, the neutralizing antibodies elicited by Sst+gag mRNA immunized mice were not ) immunized mice mouse.
From the perspective of the 333rd day of the immune endpoint , the neutralizing antibody titers targeting the original strain, Beta and Omicron triggered by Sst+gag mRNA immunized mice were higher than those of mice inoculated only with Sst mRNA or Spike mRNA (natural CTD) .
Interestingly , for targeting Omicron subtypes BA.2.12.1 and BA.4, mice inoculated with Spike mRNA (natural CTD) had higher neutralizing antibody titers.
Neutralizing antibodies against the original strain
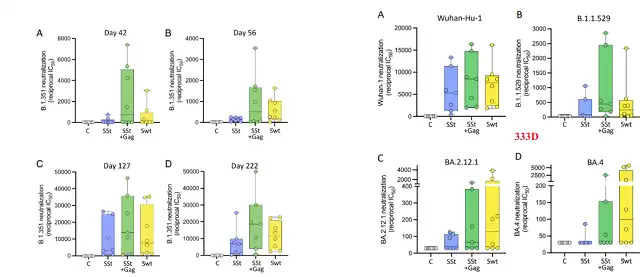
Neutralizing antibodies against mutant strains
Conclusion
The highlight of this work is the combination of gag protein-based VLP technology and mRNA technology, and simultaneously express gag mRNA and Spike protein chimeric with the CTD region of gp41 protein in host cells to promote efficient assembly and release of VLP by host cells.
Theoretically, since VLP-Spike is more similar to natural virions in particle size, shape, and antigen presentation, it should be able to stimulate a stronger B cell response.
However, judging from the immune data obtained in this article, it can only show that the gag VLP-Spike mRNA vaccine has a slight advantage over the traditional Spike mRNA vaccine to a certain extent. For some mutant strains, it is not as good as expressing the natural Spike antigen mRNA vaccines.
Previously, we introduced an article published on Cell that Spike protein recruits ESCRT, releases virus-like particles, and enhances the immune effect of mRNA vaccines . It also uses mRNA technology to generate VLPs.
They did not introduce additional viral proteins , but incorporated The ESCRT- and ALIX-binding domain (EABR) inserts into the cytoplasmic tail region of the S protein, thereby recruiting the ESCRT protein and inducing eVLP budding from the host cell.
Compared with the conventional mRNA vaccine encoding Spike protein, it can greatly enhance the antibody response.
In terms of immune effect and innovation, ESCRT-based VLP mRNA vaccines are superior to gag protein-based VLP mRNA vaccines.
Co-express gag protein and chimeric Spike to develop VLP COVID-19 mRNA vaccine
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



