CAR-T therapy brings great opportunities in the cellular immune market
- A Single US$2.15-Million Injection to Block 90% of Cancer Cell Formation
- WIV: Prevention of New Disease X and Investigation of the Origin of COVID-19
- Why Botulinum Toxin Reigns as One of the Deadliest Poisons?
- FDA Approves Pfizer’s One-Time Gene Therapy for Hemophilia B: $3.5 Million per Dose
- Aspirin: Study Finds Greater Benefits for These Colorectal Cancer Patients
- Cancer Can Occur Without Genetic Mutations?
CAR-T therapy brings great opportunities in the cellular immune market
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- What is the difference between Atorvastatin and Rosuvastatin?
- How long can the patient live after heart stent surgery?
CAR-T therapy brings great opportunities in the cellular immune market. Cellular immunotherapy represented by CAR-T leads anti-cancer therapy into a new era. CAR-T therapy has entered the public eye due to its excellent curative effect in the field of hematological tumors, and at the same time it has led the anti-cancer treatment into a new era.
Immunotherapy allows us to see the dawn of victory in the war against cancer for the first time. Different from traditional cancer treatment drugs, CAR-T cell therapy mainly uses T cells to activate the body’s natural host defense mechanism.
CAR-T can recognize tumor antigens without the restriction of major histocompatibility complex (MHC), can recognize MHC-independent targets, and at the same time enhance the lethality of T cell immunity through costimulatory molecular signals, thereby overcoming tumor Cells down-regulate the expression of MHC or inhibit the secretion of co-stimulatory molecules to cause immune escape.
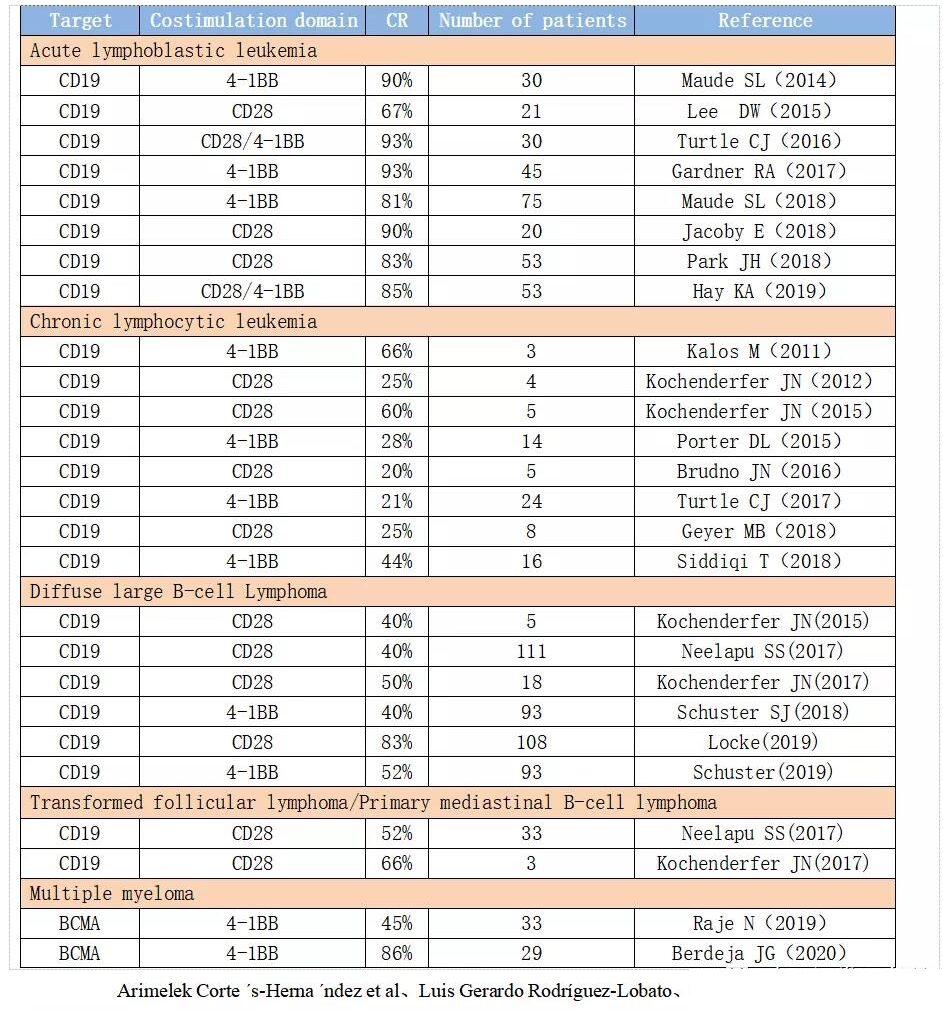
CAR-T shows a high clinical remission rate, especially for hematological tumors of specific targets. For cancer patients who are helpless with traditional treatment methods, CAR-T provides a new treatment method and has demonstrated breakthrough efficacy. Clinical trials of autologous anti-CD19-CAR-T cells have been obtained in adults and children with r/r ALL.
With a remission rate of up to 93%, clinical trials using CAR-T cells in r/r CLL patients showed that the total effective rate (ORR) was as high as 75%, and the complete remission (CR) was as high as 66%. Some patients with lymphoma also exhibit unprecedented remission rates.
According to the currently completed and ongoing CAR-T clinical trials around the world, CD19 is used as the target for nearly half, and CD19 has become the most important molecular biomarker for CAR-T immunotherapy.
The cellular immunity industry welcomes market opportunities and addresses major unmet clinical needs. The immunotherapy represented by CAR-T therapy has achieved breakthrough progress, bringing new hope to cancer patients, especially the introduction of new mechanisms such as centralized procurement and medical insurance catalog negotiations, which significantly enhance patients’ willingness and ability to pay, and cellular immunity will improve Gradually solve the current unmet major clinical needs, while at the same time the Chinese cellular immune industry is also ushering in major market opportunities.
According to the Frost & Sullivan report, the market size of China’s cellular immunotherapy products is expected to rise from RMB 1.3 billion to 10.2 billion from 2021 to 2023, with a compound annual growth rate of 181.5%. The market is expected to reach RMB 58.4 billion in 2030.
At present, CAR-T therapy is mainly aimed at hematological tumors. Therefore, this article mainly analyzes the market for three indications of acute lymphoblastic leukemia, diffuse large B-cell lymphoma and multiple myeloma, including its market scale calculation and competitive landscape analysis.
Problems and challenges of cellular immunotherapy. Although CAR-T has made breakthrough progress in hematological tumors, there are still toxic side effects that cannot be ignored during the treatment of patients. These side effects may be serious or even fatal, especially cytokine release syndrome and Neurotoxicity, etc.; due to the suppression of the tumor microenvironment and the lack of specific tumor-related antigens, CAR-T has not been satisfactory in the treatment of solid tumors so far; due to the complexity of the manufacturing process and the relatively high With a long production cycle and lack of standardized supervision, CAR-T is still a highly personalized therapy.
The most important thing is that most cellular immunotherapy is modified by patient’s autologous cells. It is a non-universal cellular immunotherapy and faces high costs. Manufacturing cost, according to the current manufacturing process, the total cost of producing CAR-T products is between 150,000 and 300,000 US dollars. Reducing the production cost of cell products is a problem that must be solved in the process of CAR-T industrialization.
Investment Advice:
Cellular immunotherapy represented by CAR-T has shown excellent efficacy in the field of hematological tumors. With the gradual listing of related products in China, CAR-T therapy is bound to occupy a place in the future. At the same time, CAR-T, as a new technological path, can be said to open up a new treatment approach against cancer.
In the future, it may completely change the traditional treatment of cancer. At present, dual-target CAR, CAR-T combined with PD-1, CAR -NK, UCAR-T, TCR-T and other directions are all placed high hopes in the industry. We have reason to believe that there will be new technological breakthroughs in this field in the future.
It is recommended to pay attention to related companies in the Chinese CAR-T field that have faster product progress and strong industrialization capabilities: such as Nanjing Legend, WuXi Junuo, Fosun Kate, Keji Bio, etc.
Risk warning:
Risks that the R&D progress is less than expected; commercialization risks; policy risks; and new technology substitution risks.
Report Contents:
1. Introduction and development history of CAR-T therapy
Adoptive cellular immunotherapy includes non-specific immunotherapy LAK, CIK, DC-CIK, NK and specific tumor infiltrating lymphocytes (TIL), CAR-T and TCR-T. CAR-T therapy has entered the public eye due to its excellent curative effect in the field of hematological tumors, and at the same time has led the anti-cancer treatment into a new era. Immunotherapy allows us to see the dawn of victory in the war against cancer for the first time.
Chimeric Antigen Receptor T-Cell Immunotherapy (CAR-T) is a single-chain variable region antibody that encodes an immunoglobulin derived from an antibody that is used to recognize tumor cell antigens (Scfv) the heavy chain variable region (VH) and light chain variable region (VL) of the recombinant protein gene and the DNA chain of the T cell killing activation signal CD3ζ are connected to the genetic engineering expression vector through gene recombination technology, and It is expressed on the killer T cell membrane to form a chimeric antigen receptor, so that T cells do not rely on MHC-I to recognize tumor cells. After purification, in vitro expansion and activation, CAR-T cells are returned to the patient’s body.
Make it specifically perform the function of killing tumor cells. CAR carrier is mainly composed of three parts: antigen binding domain, transmembrane domain and intracellular signal transduction domain.
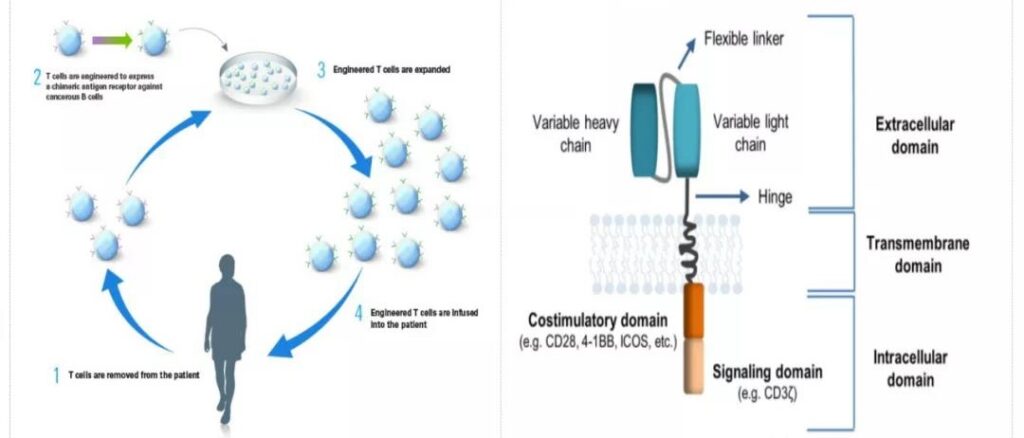
In 2012, in a CAR-T clinical trial for children with B-cell acute lymphoblastic leukemia conducted by Carl June, a child with acute leukemia named Emily was able to target CD19 when the second relapse was incurable. After the CAR-T treatment, the tumor completely disappeared after three weeks.
Emily is still alive and healthy. It can be said that CAR-T has created a medical miracle in human history and brought new hope to countless cancer patients.
In 2017, the US FDA approved the marketing of two CAR-T cell therapies targeting CD19, namely Novartis’ Kmriah (tisagenlecleucel) and Kite’s Yescarta (axicabtagene ciloleucel) for the treatment of acute lymphoblastic leukemia in children and adolescents, respectively. leukemia (ALL) and specific types of non-Hodgkin lymphoma (NHL).
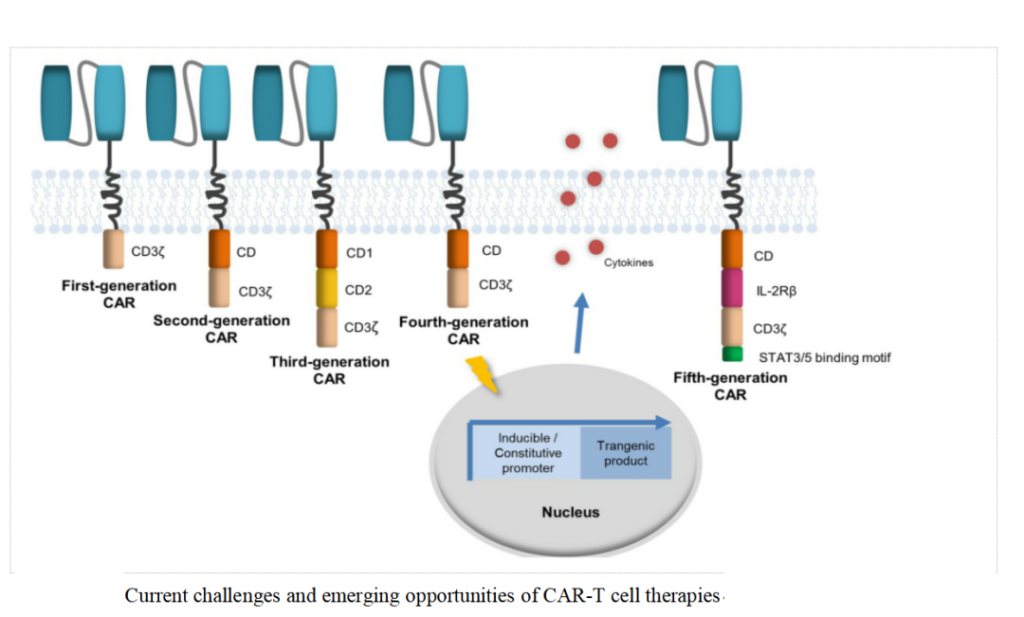
CAR-T therapy has developed to the fifth generation so far: The first generation CAR only relies on CD3ζ to mediate T cell activation. Due to the lack of intracellular costimulatory signals, it cannot provide long-term T cell expansion signals and continuous anti-tumor.
Effect, the clinical curative effect is limited; in order to improve the proliferation activity of T cells and prolong their survival time, one or two costimulatory molecules (CD28, 4-1BB, ICOS or OX40, etc.) are added to the intracellular signal transduction area.
In the second and third generation CARs, among the existing costimulatory domains, CD28 and 4-1BB are the most frequently used costimulatory molecules. In contrast, 4-1BB is used as a costimulatory signal for CAR-T cells.
The expansion in the body is more gentle and lasting, the risk of cytokine storm is lower, and the recurrence rate is lower; the fourth-generation CAR introduces pro-inflammatory cytokines (IL-12, IL-15, IL-18, etc.) and co- The main purpose of stimulating ligands is to overcome the suppression of the tumor immune microenvironment.
For example, a cytokine that is often overexpressed in CAR-T cells is IL-12, which enhances the secretion of IFNγ, granzyme B and perforation by T cells.
The effective molecule of cytokine allows NK cells to be recruited to eliminate tumor cells not recognized by CAR; the fifth-generation CAR is based on the second-generation, adding costimulatory domains that activate other signaling pathways, such as IL2-2Rβ intracellular Combining the domain of SAAT3/5, etc.
At present, most of the company’s products are based on the second-generation CAR-T technology, and explore and develop on this.
2. CAR-T highlights the advantages of treatment, and some cancers show significant curative effects
For cancer patients who are helpless with traditional treatment methods, CAR-T provides a new treatment method and has demonstrated breakthrough efficacy.
Clinical trials of autologous anti-CD19-CAR-T cells have been obtained in adults and children with r/r ALL.
With a remission rate of up to 93%, clinical trials using CAR-T cells in r/r CLL patients showed that the total effective rate (ORR) was as high as 75%, and the complete remission (CR) was as high as 66%.
Some patients with lymphoma also exhibit unprecedented remission rates.
Different from traditional cancer treatment drugs, CAR-T cell therapy mainly uses T cells to activate the body’s natural host defense mechanism. CAR-T can recognize tumor antigens without the restriction of major histocompatibility complex (MHC), can recognize MHC-independent targets, and at the same time enhance the lethality of T cell immunity through costimulatory molecular signals, thereby overcoming tumor Cells down-regulate the expression of MHC or inhibit the secretion of co-stimulatory molecules to cause immune escape.
CAR-T therapy has made significant progress in hematological tumors, especially in CD19-CAR-T. CD19 is expressed in most B-line lymphoma cells, but not in hematopoietic stem cells and normal tissues. In the ongoing CAR-T clinical trials, CD19 is used as the target for nearly half, and CD19 has become the most important molecular biomarker for CAR-T immunotherapy.
3. CAR-T therapy shows great power, and the cellular immune industry ushered in market opportunities
The immunotherapy represented by CAR-T therapy has achieved breakthrough progress, bringing new hope to cancer patients, especially the introduction of new mechanisms such as centralized procurement and medical insurance catalog negotiations, which significantly enhance patients’ willingness and ability to pay, and cellular immunity will improve Gradually solve the current unmet major clinical needs, while at the same time the Chinese cellular immune industry is also ushering in a major market opportunity.
According to the Frost & Sullivan report, the market size of China’s cellular immunotherapy products is expected to rise from RMB 1.3 billion to 10.2 billion from 2021 to 2023, with a compound annual growth rate of 181.5%. The market is expected to reach RMB 58.4 billion in 2030.
In 2017, NMPA included cell therapy into therapeutic biological products for application management. In the same year, it issued the “Technical Guidelines for Research and Evaluation of Cell Therapy Products (Trial)”.
At present, CAR-T therapy is the most effective in the field of cellular immunotherapy, and the indications are concentrated on hematological tumors. At present, major clinical breakthroughs are mainly relapsed/refractory acute B lymphocytic leukemia (ALL), relapsed/refractory diffuse Large B-cell lymphoma (DLBCL) and relapsed/refractory multiple myeloma (MM), etc.
Hematological tumors are diseases of the hematopoietic system including lymphoma, leukemia and multiple myeloma, etc., which have the characteristics of high malignancy, complex treatment and poor prognosis.
Different from solid tumors, hematological tumors are mainly treated with radiotherapy and chemotherapy combined with bone marrow transplantation.
As targeted therapy and immunotherapy bring new options to the clinic, the survival time of patients has also been significantly improved.
According to a 2016 report on the global burden of disease research, the number of new cases and deaths of hematological tumors worldwide each year are 1.14 million and 460,000, respectively, accounting for 7% and 5% of all tumors, respectively.
3.1 Acute lymphocytic leukemia
Acute lymphocytic leukemia (ALL) is a common hematological malignancy, characterized by abnormal proliferation and aggregation of immature lymphocytes in bone marrow and lymphoid tissues, with diverse biological characteristics and high clinical heterogeneity Sex.
Acute lymphocytic leukemia can be seen in both children and adults, but its peak incidence is between the ages of 2 and 5 years. After that, the age increases gradually and the incidence rate increases slightly after the age of 50.
Among all leukemias, ALL accounts for 15%, and about 30%-40% of acute leukemias. ALL is the most common malignant tumor in children.
Regarding the treatment of ALL, the first stage is mainly chemotherapy. Before the leukemia cells have developed drug resistance, chemotherapy drugs are used to maximize the killing of leukemia cells and restore the normal hematopoietic function of the bone marrow.
In this stage, the chemotherapy drugs mainly use VDLP (D) , Namely vincristine, daunorubicin, asparaginase, prednisone or dexamethasone. With the continuous improvement of chemotherapy regimens, the overall survival rate of ALL patients has reached 90%, and the long-term disease-free survival rate has reached 80%.
For patients with clear molecular biological abnormalities, targeted therapy drugs can be given to improve the prognosis of patients. Conditions can be performed on patients with hematopoietic stem cell transplantation (HSCT), so as to finally achieve clinical cure.
Although the survival rate of ALL patients has improved significantly in recent years, there are still 20%-30% of relapsed and refractory patients. Their long-term survival rate is generally low (less than 40%). For these patients, new drugs or therapies are still needed .
Especially for relapsed and refractory ALL without molecular targeted drug therapy, the immunotherapy program represented by CAR-T may be the best choice at present.
3.2 Diffuse large B-cell lymphoma
Lymphomas are classified into Hodgkin’s lymphoma (HL) and non-Hodgkin’s lymphoma (NHL) according to the tumor cells, of which NHL accounts for 80%-90%, and can be divided into B cell type, T cell type and cell source. NK/T cell type. Among them, 70%-80%% are B cell lines, a few are T cell lines, and NK cells are even rarer. Among B-cell lymphomas, diffuse large B-cell lymphoma accounts for 30%-40%. Diffuse large B-cell lymphoma (DLBCL) is the most common type of NHL.
Regarding treatment options for DLBCL patients, the standard first-line therapy is rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP). Approximately 60-70% of DLBCL patients can be effectively relieved with this program. However, approximately 30-40% of patients will relapse, or in a small number of patients, R-CHOP therapy is refractory. If the patients meet the conditions, hematopoietic stem cell transplantation is recommended, but a considerable number of patients cannot be cured by this method.
In general, there are still many patients with relapsed/refractory DLBCL who cannot meet their medical needs due to unqualified transplantation or refractory chemotherapy. For these patients, it is necessary to adopt a new program. CAR-T has shown great promise as a cell therapy using autologous genetically modified T cells, and has been approved by the FDA for the subsequent treatment of r/r DLBCL.
According to the White Paper on Survival Report of Lymphoma Patients in 2019, lymphoma patients have a strong demand for new drugs and treatment methods. Among them, CAR-T therapy is the most anticipated by patients, but new drugs and therapies are bringing treatment to patients The benefits also bring a considerable economic burden to patients. According to the report statistics, the total expenditure of lymphoma patients is about 326,000 yuan, and the self-pay ratio is about 60%.
Assuming that the annual cost of Chinese CAR-T treatment of ALL patients is 300,000 yuan, and the Chinese penetration rate of CAR-T is 30% and 40%, the market space for ALL is 704 million yuan and 939 million yuan, respectively.
There are many CAR-T companies targeting B-NHL indications in China. Among them, Fosun Kate and WuXi Giant Nuo have submitted their CAR-T product listing applications. Considering that Novartis has products listed abroad, it only needs to do it in China. The bridge test is expected to be the third Chinese CAR-T company to submit a listing application. The second tier is the Chinese companies represented by Hengrun Dasheng and Keji Biological, both of which are currently in clinical phase I.
3.3 Multiple myeloma
Multiple myeloma (MM) is a malignant disease in which clonal plasma cells proliferate abnormally. It is the second most common hematological malignancy after non-Hodgkin’s lymphoma.
For newly-treated patients, sequential autologous hematopoietic stem cell transplantation and maintenance therapy with a combination regimen containing protease inhibitors, immunomodulators, steroid hormones, and monoclonal antibodies is the current standard treatment.
Since it is not yet possible to completely eliminate minimal residual disease (MRD) and eventually relapse cannot be avoided, most patients will eventually face the problem of recurrence or disease progression.
For patients with relapse or refractory (r/rMM), it is the choice to follow the past Drugs with different therapeutic mechanisms of action.
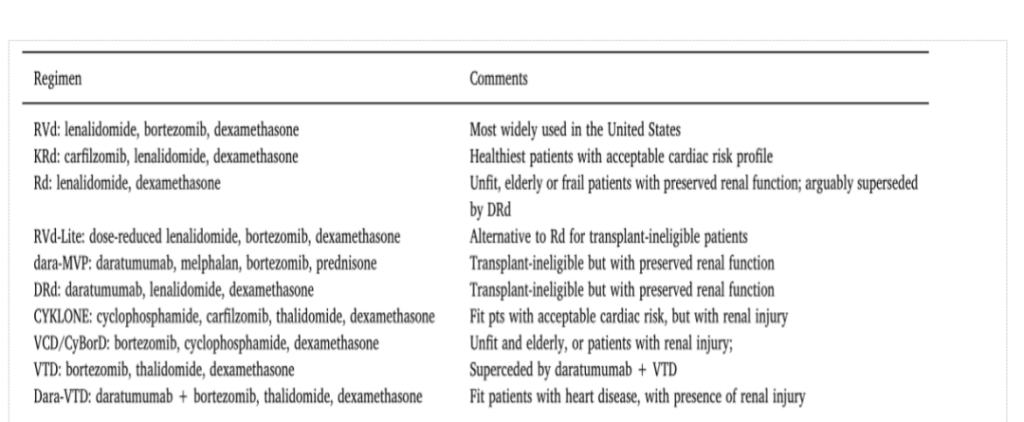
Regarding the drug market for multiple myeloma, a market with six major varieties of competition including lenalidomide, pomalidomide, darelimumab, bortezomib, carfilzomib and erotuzumab has been formed pattern.
The emergence of immunotherapies represented by BCMA CAR-T, as well as the emergence of new therapies such as Belantamab mafodotin, XPO1 inhibitors, PD-1, etc., will change the drug pattern of multiple myeloma in the future.
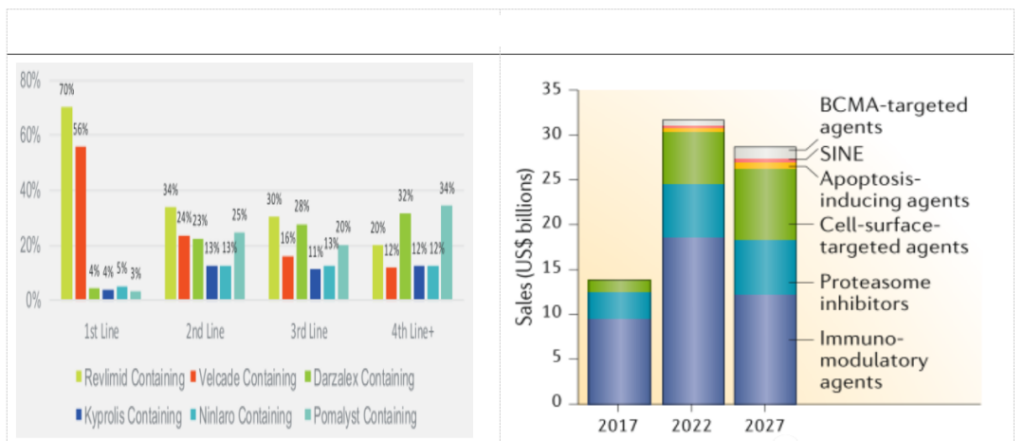
Combining the epidemiology of multiple myeloma and its drug pattern, we estimate the market space for relapsed and refractory multiple myeloma, assuming that the annual cost of CAR-T treatment for MM patients in China is 300,000 yuan, and relapsed and refractory MM The ratio of CAR-T is 60%. When the Chinese penetration rate of CAR-T is 20% and 30%, the market space for r/r MM is 1.966 billion yuan and 2.621 billion yuan respectively.
BCMA is an extremely important B cell biomarker, which is widely present on the surface of MM cells. In recent years, it has become a very popular immunotherapy target for MM and other hematological malignancies. CAR-T therapy for BCMA targets has shown excellent clinical efficacy and is expected to become an important treatment for r/r MM in the future.
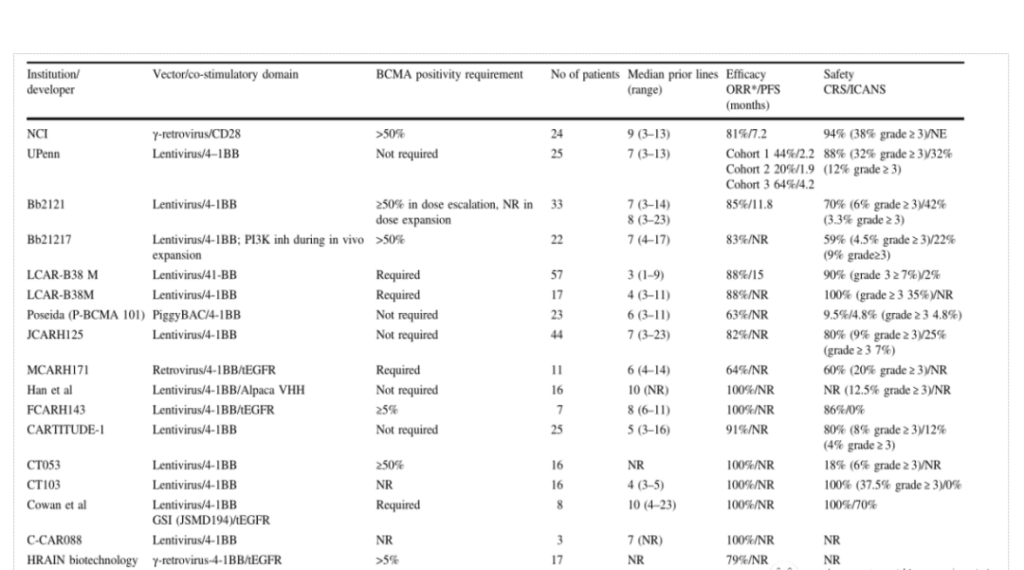
At present, there is no CAR-T product on the market for MM indications in the world. Bluebird’s bb2121 is expected to become the world’s first BCMA-CAR-T product for MM. The Chinese CAR-T company Nanjing Legend follows closely. According to current clinical data, its efficacy is not inferior to bb2121, and it has been granted breakthrough therapy certification by the FDA and CDE. It can be said that the Chinese company Nanjing Legend has ranked first in the world in the BCMA target. , Other Chinese companies such as Keji Biology, Hengrun Dasheng, Nanjing Reindeer, etc. are in the second echelon.
4. Problems and challenges in cellular immunotherapy
4.1 Toxic and side effects
Although CAR-T has made breakthrough progress in hematological tumors, there are still toxic side effects that cannot be ignored during the treatment of patients, and these side effects may be serious or even fatal.
Cytokine Release Syndrome (CRS) is one of the most common side effects of cellular immunotherapy.
After CAR-T cells are reinfused into the patient’s body, the T cells in the patient’s body will be activated and rapidly increase in value, triggering the release of a large number of cytokines (such as TNFα, IL-2, IL-6, IL-8, etc.), thereby causing the patient to have a high fever , Fatigue, myalgia, dyspnea, arrhythmia and other inflammatory reactions, which severely lead to multiple organ failure. Within 3 weeks after CAR-T cell infusion, if one of the following symptoms occurs, CRS should be considered:
①body temperature ≥38 ℃;
②hypotension (systolic blood pressure <90 mmHg);
③hypoxemia (arterial oxygen Saturation <90%);
④ Toxic reaction of organs.
Generally, the severity of CRS is closely related to tumor burden. CRS occurs within one week after CAR-T cell therapy, and 30-94% of patients will have varying degrees of CRS. CRS is divided into different levels according to different symptoms.
The American Society of Blood and Bone Marrow Transplantation (ASBMT) has formulated a new definition and classification of CRS and neurotoxicity related to immune cell therapy.
Neurotoxicity, also known as CAR-T cell-related encephalopathy syndrome (CRES), is the second most common life-threatening event associated with CAR-T cell therapy.
CAR-T cell therapy is often observed in the cerebrospinal fluid of patients -T cells may be related to the release of a large number of cytokines, which increases the permeability of blood vessels and blood-brain barrier.
A small number of patients will experience severe neurotoxicity after receiving CAR-T cell therapy. The main clinical manifestations are confusion, aphasia, lethargy, tremor, and language disorders. In severe cases, seizures and cerebral edema may occur.
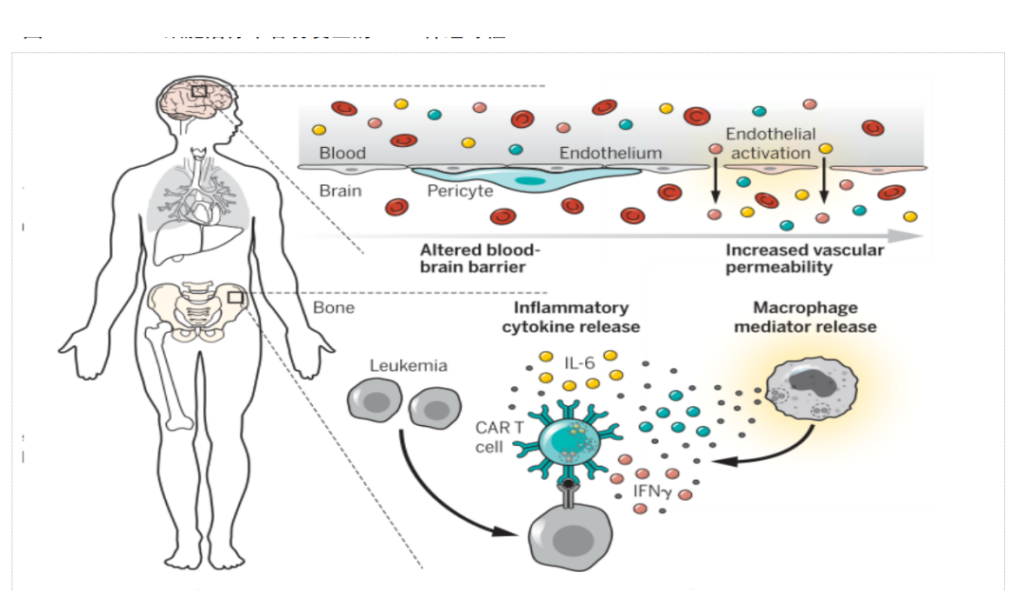
Clinically, it has been shown that most mild CRS and CRES can be relieved on their own, but for CRS and CRES greater than or equal to Grade 2 intervention treatment is required, such as medical testing, auxiliary oxygen inhalation, anti-IL-6 monoclonal antibody and the use of steroid hormones, etc.
In addition to the above-mentioned toxic and side effects, there will be off-target effects, allergic reactions, potential tumorigenic risks during viral transfection, and tumor recurrence during CAR-T treatment. These are all clinical factors that limit the application of CAR-T cells.
4.2 Poor efficacy of solid tumors
The curative effect of CAR-T has been fully verified in hematological tumors such as ALL, DLBCL and MM, but so far, the therapeutic effect of CAR-T in solid tumors is not satisfactory. The reason for the poor anti-tumor effect in the treatment of solid tumors may be due to Many aspects, such as the suppression of the tumor microenvironment and the lack of specific tumor-associated antigens.
The problem of target selection for solid tumors. Due to the inherent heterogeneity of solid tumors, it is difficult to find a target antigen that can cover all tumor cells in the same tumor.
The lack of tumor-specific antigen (TSA) severely limits the application of CAR-T. Targeting tumor-associated antigen (TAA) is an alternative method to overcome the deficiency of TSA, but tumor-associated antigens are generally expressed in tumor and normal tissues at the same time, so patients may face the potential risk of targeted non-tumor toxicity when CAR-T treatment In addition, traditional CAR-T therapy needs to recognize cell surface antigens, but only about 1% of targetable proteins are expressed on the cell surface, which means that a large number of potential tumor target antigens are not suitable for CAR-T therapy, TCR -T and Nanobody CAR-cell therapy is a potential solution.
It is difficult for CAR-T cells to infiltrate tumor tissues effectively. Different from hematological tumors, after the CAR-T is injected back into the patient’s body, the CAR-T cells need to pass through the extracellular matrix and overcome the immunosuppressive microenvironment to reach the solid tumor site.
The occurrence of tumors will make the cell surface and Insufficient chemokines secreted by the tumor microenvironment, as well as the characteristics of solid tumors, such as concentrated blood vessels, tumor-related fibroblasts, and extracellular matrix formed by bone marrow cells, all increase the difficulty of T cell infiltration at the tumor site.
Will cause CAR-T cells to not effectively infiltrate tumor tissues and kill tumor cells.
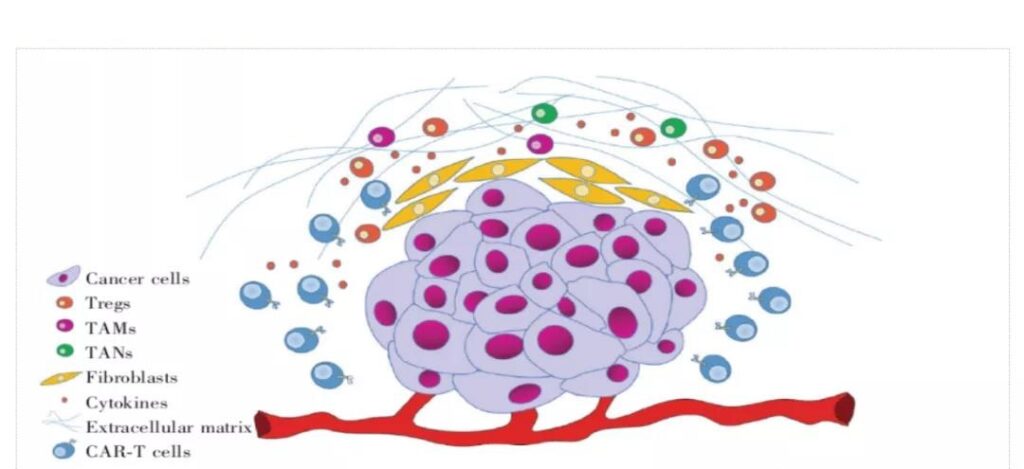
Inhibition of the tumor microenvironment of solid tumors. Even if CAR-T enters the solid tumor, it will face the suppression of the internal tumor microenvironment (TME).
TME is a complex system composed of various cells, signal molecules, soluble factors, fibrotic extracellular matrix and immune regulatory cells. TME has the characteristics of oxidative stress, nutrient depletion, acidic pH environment and hypoxia, which will reduce the activity of CAR-T cells.
The immune regulatory cells in TME include regulatory T cells (Tregs), tumor-associated macrophages (TAMs) and tumor-associated neutrophils (TANs).
TAMs and TANs can be combined with Tregs to produce immunosuppressive cytokines and related ligands, such as TGF-β, PD-L1, etc., which will reduce the activity of CAR-T cells; indoleamine 2,3-double produced by immune regulatory cells Oxygenase (IDO) can decompose tryptophan into kynurenine, causing GCN2 activation and mTOR inhibition, resulting in decreased reactivity of CAR-T cells and Tregs, making CAR-T cells unable to effectively treat IDO-expressing tumors.
4.3 CAR-T is facing difficulties in industrialization, and production costs remain high
Although the clinical safety and effectiveness of CAR-T have been verified, due to the complexity of its manufacturing process, long production cycle and lack of standardized supervision, CAR-T is still a highly personalized therapy. . Regarding the CAR-T production process, it is mainly divided into the following steps:
1. Apheresis of white blood cells and cell washing. Use density gradient centrifugation to separate white blood cells from the peripheral blood of the patient, and then wash to remove the anticoagulant added during the white blood cell separation process;
2. Enrich and activate T cells. T cells are separated by CD4/CD8 specific antibodies or surface-labeled coupled magnetic beads, and then CD3/CD28 monoclonal antibody magnetic beads or artificial antigen-presenting cells can be added to activate T cells in the cell culture medium;
3. Gene delivery/transfection. Use lentiviral/retroviral vectors or non-viral methods (electrotransfection DNA, transposon/transposon system) to transfect CAR into T cells;
4. Cell culture. Expand and culture T cells through cell culture equipment such as bioreactors;
5. Freeze and transport, and finally return to the patient. Adjust the cell number and medium composition according to the formula, then transfer the product to a suitable container for freezing and transportation, and finally return to the patient.
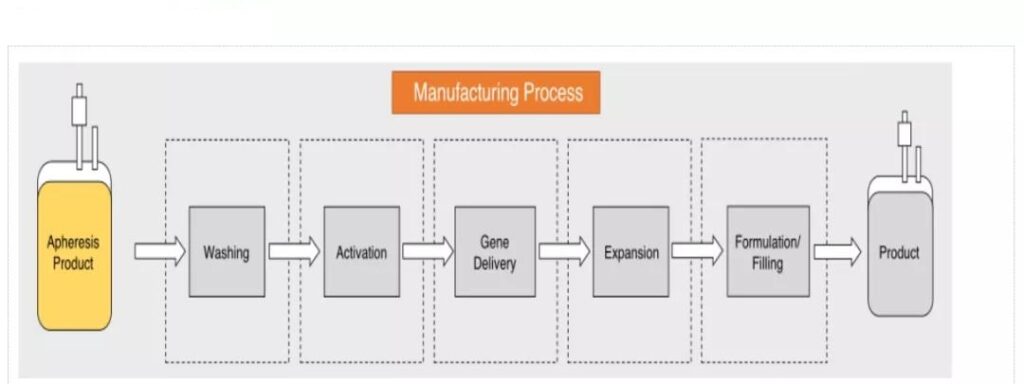
Unlike traditional drugs, CAR-T is a highly personalized cellular immunotherapy technology with a long production cycle, complex preparation process, and numerous quality control links.
The entire production process involves plasmid preparation, virus preparation, sample collection, and reception. , Processing, cell stimulation, transduction/transfection, amplification, harvesting, quality inspection, cryopreservation and transportation, etc., manufacturers need to ensure strict quality control in each link.
As the most important raw material in CAR-T, the quality control of viruses and plasmids is very critical. Manufacturers need to ensure the quality and safety of transfected viruses, and at the same time, the stability of the production process, process parameters and quality control are the same throughout the production process Importantly, these all put forward higher requirements for the industrialization capabilities of CAR-T companies.
The most important thing is that most cellular immunotherapies are modified from patients’ autologous cells. They are non-universal cellular immunotherapy and face high manufacturing costs.
According to the current manufacturing process, the total cost of producing CAR-T products is between US$150,000 and US$300,000. Reducing the production cost of cell products is also a problem that must be solved in the process of CAR-T industrialization.
The French company Cellectis announced in 2017 that its UCAR-T123 has obtained IND approval from the US FDA. This is the world’s first universal CAR-T product. It can select T cells from allogeneic healthy people and use gene editing technology to modify them, and then Applied directly to patients, the product has the possibility of large-scale production, which may achieve large-scale and greatly reduce the production cost of CAR-T.
Currently, UCAR-T companies globally include Allogene, CRISPR Therapeutics, Celyad, Precision BioSciences, etc. At present, general CARs are in the clinical stage, and allogeneic CAR-T still faces some problems, such as graft-versus-host disease (GVHD) and allogeneic CAR-T cells may be cleared by the host’s immune system, limiting their anti-tumor activity, etc.
Cellectis’ UCAR-T123 clinical trial was once stopped by the FDA due to the death of subjects, and then restarted after modifying the clinical protocol. Recently, its clinical trial of UCAR-T CS1A for multiple myeloma caused one The patient developed CRS and died of cardiac arrest 25 days after the treatment.
After that, the FDA urgently stopped it. It can be seen that there are still many problems to be solved for the general CAR-T, and the road ahead will not be smooth sailing.
5. Risk warning
Risks that the R&D progress is less than expected; commercialization risks; policy risks; new technology substitution risks.
CAR-T therapy brings great opportunities in the cellular immune market
CAR-T therapy brings great opportunities in the cellular immune market
CAR-T therapy brings great opportunities in the cellular immune market
(source:internet, reference only)
Disclaimer of medicaltrend.org



