Reveal the quantitative law of bacterial cell wall growth
- WHO Releases Global Influenza Vaccine Market Study in 2024
- HIV Infections Linked to Unlicensed Spa’s Vampire Facial Treatments
- A Single US$2.15-Million Injection to Block 90% of Cancer Cell Formation
- WIV: Prevention of New Disease X and Investigation of the Origin of COVID-19
- Why Botulinum Toxin Reigns as One of the Deadliest Poisons?
- FDA Approves Pfizer’s One-Time Gene Therapy for Hemophilia B: $3.5 Million per Dose
Reveal the quantitative law of bacterial cell wall growth
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- What is the difference between Atorvastatin and Rosuvastatin?
- How long can the patient live after heart stent surgery?
Reveal the quantitative law of bacterial cell wall growth.
Nature Communications published online a collaborative research paper “Probing bacterial cell wall growth by tracing wall-anchored protein complexes” by the Peking University Biomedical Frontier Innovation Center (BIOPIC) and the Bai Fan Group of the School of Life Sciences. Cell wall growth law)”.
The growth and division of cells are important life processes. It is very important to maintain a stable cell shape during cell growth and division, which is often achieved by sophisticated regulatory mechanisms.
In bacteria, the shell structure of a cell is composed of a cell membrane (mainly containing phospholipid molecules) and a cell wall (mainly containing peptidoglycan molecules).
In addition to maintaining the shape of the cell, the shell structure of bacteria also provides mechanical support for balancing the osmotic pressure.
During the growth and division of bacteria, under the action of cytoskeleton proteins and a series of enzymes, the bacterial cell wall undergoes dynamic changes such as extension, remodeling, and breakage.
Although the chemical composition and microscopic connection of peptidoglycan molecules have been fully studied, there are still many unknowns about how the synthesis and insertion of peptidoglycan molecules at the cellular level can change the morphology of bacterial cell walls.
Previous studies have initially realized the qualitative measurement of cell wall insertion patterns by introducing fluorescent D-type amino acids into bacterial cell wall synthesis.
However, there are still big challenges in analyzing the quantitative laws of bacterial cell wall synthesis with high temporal and spatial resolution in living cells.
In this study, the research team made innovative thinking and indirectly inferred the dynamic law of cell wall growth by tracking the relative movement of bacterial cell wall anchoring proteins.
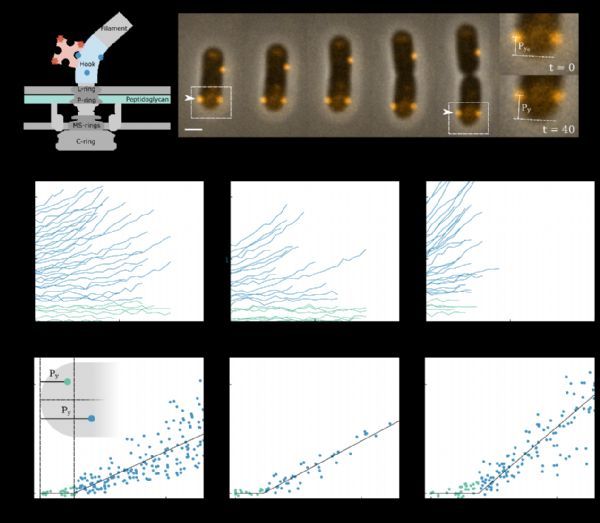
The researchers used Alexa 594 fluorescent dye to fluorescently label the bacterial flagellar motor (BFM).
BFM is a typical protein complex fixed on the bacterial cell wall, and there are 5-10 BFMs distributed in a cell.
Because they are tightly fixed to the cell wall, the growth of the bacterial cell wall can be resolved by monitoring changes in the relative distance between these fluorescent “anchors” on living cells (Figure 1A, Figure 2A-B).
The researchers observed the change in the distance between the fluorescent BFM and one end of the bacteria during the growth of the bacteria.
Interestingly, there seems to be a clear boundary in the extension of the bacterial cell wall, separating the “active zone” where peptidoglycan molecules are actively inserted and the “quiescent zone” where no peptidoglycan molecules are inserted.
The experimental evidence supporting this conclusion is that with the growth of bacterial cells, the BFM beyond the “boundary distance” from the endpoint will gradually move away from the endpoint, while the BFM within the “boundary distance” from the endpoint always maintains the same distance from the endpoint (Figure 1B-D).
The researchers monitored the changes in the relative distance between the fluorescent BFMs during the growth of the bacteria (Figure 2A-B).
Interestingly, in the direction of bacterial extension, the rate of change of the relative distance between the two fluorescent BFMs is positively correlated with the relative distance itself; while perpendicular to the direction of bacterial extension, there is no relative distance between the two fluorescent BFMs.
Change (Figure 2C-F). This shows that along the direction of bacterial growth, the insertion and extension of peptidoglycan molecules in the “active zone” are spatially uniform, and there will be no “twisting” perpendicular to the direction of growth during bacterial growth.
After the insertion and extension of peptidoglycan molecules in the known “active zone” are spatially uniform, the researchers took the cell center as the origin and introduced a new normalized coordinate system (the coordinate of the fluorescent BFM relative to the cell center divided by the cell length).
In this normalized coordinate system, when the bacteria grow without dividing, the coordinates of each fluorescent BFM are constant.
The researchers tracked the bacteria undergoing cell division and found that the spatial position of the fluorescent BFM after entering the daughter cell can be predicted by a simple Bernoulli shift map model.
The researchers explored the physiological significance of bacterial cell wall anchoring proteins following the Bernoulli shift map model during cell division.
Using two-color fluorescent labeling, they monitored the spatial position of the newly generated BFM after bacterial division and found that they prefer to concentrate in the middle of the bacteria, which may be related to the position of the gene transcribing BFM on the bacterial DNA that is biased toward the middle.
However, an important feature of the Bernoulli shift map is that it can quickly erase the unevenness of the spatial distribution, so only after one cell division, it can cause the BFM to tend to a uniform spatial distribution on the bacterial surface, which is conducive to bacterial swimming and Tendency.
To sum up, this study revealed the dynamic law of cell wall growth by tracking the relative movement of bacterial cell wall anchoring proteins. It was found that:
1) During bacterial growth, there are “active areas” where peptidoglycan molecules are actively inserted in the cell wall and there is no “active area” in the cell wall. The “quiescent zone” where peptidoglycan molecules are inserted;
2) Along the growth direction of bacteria, the insertion and extension of peptidoglycan molecules in the “active zone” are spatially uniform;
3) The bacteria undergo cell division and cell wall anchoring proteins enter The spatial position behind the daughter cell follows the Bernoulli shift map model.
This research is of great significance for understanding the growth of bacterial cell walls.
(source:internet, reference only)
Disclaimer of medicaltrend.org



