Pfizer vaccine: 6-month Trial data of after COVID-19 vaccination Released!
- WHO Releases Global Influenza Vaccine Market Study in 2024
- HIV Infections Linked to Unlicensed Spa’s Vampire Facial Treatments
- A Single US$2.15-Million Injection to Block 90% of Cancer Cell Formation
- WIV: Prevention of New Disease X and Investigation of the Origin of COVID-19
- Why Botulinum Toxin Reigns as One of the Deadliest Poisons?
- FDA Approves Pfizer’s One-Time Gene Therapy for Hemophilia B: $3.5 Million per Dose
Pfizer vaccine: 6-month Trial data of after COVID-19 vaccination Released!
- Israel new drug for COVID-19: EXO-CD24 can reduce deaths by 50%
- COVID-19 vaccines for children under 12 will be available soon
- Breakthrough infection of Delta: No difference from regular COVID-19 cases
- French research: ADE occurred in Delta variant and many doubts on it
- The viral load of Delta variant is 1260 times the original COVID-19 strain
Pfizer vaccine: 6-month Trial data of after COVID-19 vaccination Released! In the past month, everyone’s confidence in controlling the epidemic through vaccines has continued to decline. Mainly from:
1. In countries like Israel, the United Kingdom, and the United States that once controlled the epidemic well through vaccines, the number of cases has skyrocketed again;
2. The first countries that initiated vaccination, such as Israel, the United Kingdom, Germany, and the United States, have introduced the third vaccine booster measures one after another;
3. Real-world data shows that the protective power of mRNA vaccines against symptomatic COVID-19 decreases as the vaccination time increases.
How accurate is the protection of mRNA vaccines?
This is the key data that is extremely important for the adjustment of vaccination strategies and epidemic prevention measures.
On July 28, 2021, C4591001 Clinical Trial Group, which united with medical centers in multiple countries, reported the data of a large follow-up cohort study 6 months after Pfizer/BioNTech’s BNT162b2 vaccination. The data was uploaded to medRxiv.
This placebo-controlled, multi-country, multi-center critical effectiveness (VE) study followed up 44,165 vaccinators over 16 years old and 2,264 vaccinated 12-15 years old.
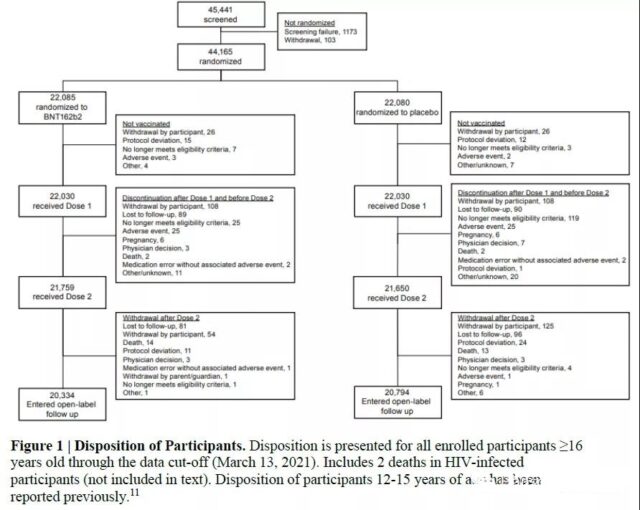
(Follow-up form. Picture source: original text)
The study found that during the 6-month follow-up period of vaccination, the effectiveness of protection was 91% (95% CI 89.0-93.2); in different countries and regions, it was 86-100% (including 100% in South Africa).
The effectiveness of protection against severe illness is 97%.
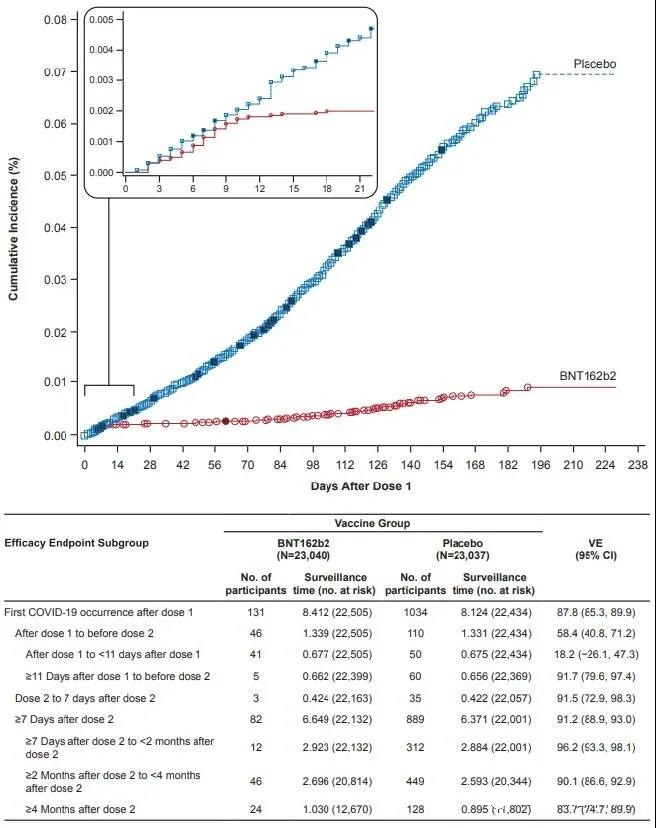
Although the overall data is gratifying, it is also necessary to see that with the extension of the second dose of vaccination, the protection of the vaccine gradually decreases; from 96.2% within 2 months of vaccination to 90.1% at 2-4 months, And 83.7% after 4 months.
In addition, during the 6-month observation period, Pfizer’s BNT162b2 vaccine showed good safety.
Expert Comments:
In medical research, the most reliable and high-level evidence is the randomized placebo-controlled study (RCT).
This Pfizer BNT162b2 vaccine RCT study showed that during the 6 months of follow-up, although the vaccine efficacy showed a gradual decline, it was still very effective in preventing COVID-19; the BNT162b2 vaccine also has good safety.
This result seems to be significantly different from the “BNT162b2 vaccine protective effect is significantly reduced” in real time. In fact, the two are not contradictory.
The RCT study compares the protective power of vaccination in two populations that are almost identical;
In the real world, high-risk groups and the elderly are the first to be vaccinated. These groups are most susceptible to COVID-19 and have a very high mortality rate. And the neutralizing antibodies induced by these people decayed fastest after vaccination. That is, real-world data is actually affected by the fact that “a very high percentage of vaccinated people are susceptible and a very high percentage of young people who have not been vaccinated” is a result of impreciseness.
You can see the real data from the epidemic situation in our county that we shared yesterday. There were zero deaths of children under 17 years of age, and 31% of elderly people over 85 died of illness.
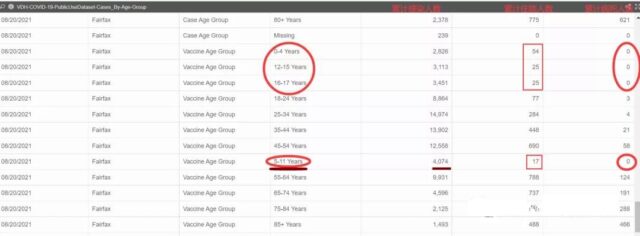
In short: the 6-month clinical trial of Pfizer’s BNT162b2 vaccine further proved the effectiveness and safety of the vaccine, which is also the basis for the official approval of the vaccine.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



