Prospects of Tolerant Dendritic Cells in Autoimmune Diseases
- A Single US$2.15-Million Injection to Block 90% of Cancer Cell Formation
- WIV: Prevention of New Disease X and Investigation of the Origin of COVID-19
- Why Botulinum Toxin Reigns as One of the Deadliest Poisons?
- FDA Approves Pfizer’s One-Time Gene Therapy for Hemophilia B: $3.5 Million per Dose
- Aspirin: Study Finds Greater Benefits for These Colorectal Cancer Patients
- Cancer Can Occur Without Genetic Mutations?
Prospects of Tolerant Dendritic Cells in Autoimmune Diseases
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Prospects of Tolerant Dendritic Cells in Autoimmune Diseases.
Differentiation and classification of dendritic cells
Dendritic cells are a heterogeneous class of innate immune cells. Major DC types include conventional DCs ( cDCs ), which can be divided into different subtypes, plasmacytoid DCs ( pDCs ), and inflammatory DCs ( infDCs ), which have distinct ontogeny, immune properties, and specific roles.

pDCs secrete large amounts of type I interferons in response to viruses, and pDCs can cross-present antigens, morphologically similar to antibody-producing plasma cells.
In humans, cDCs consist of two major subpopulations ( cDC1 and cDC2 ), initially characterized by the expression of CD141 ( BDCA3 ) and CD1c ( BDCA1 ), respectively.
Human cDC1 cells express TLR3 and TLR10, recognize viral and intracellular antigens, and produce type III interferons. cDC2 cells express TLR2, TLR4, TLR5, TLR6 and TLR8.
Another group of dendritic cells is represented by so-called inflammatory dendritic cells, which differentiate in tissues exuded from peripheral blood. InfDCs can be detected in different tissues in steady state, especially in inflammatory state.

The expression profiles and surface markers of different DC groups differ not only between species, but also in different tissues of the same organism.
The role of dendritic cells in peripheral immune tolerance
Dendritic cells recognize a large number of pathogen-associated molecular patterns ( PAMPs ) and damage-associated molecular patterns ( DAMPs ) through membrane-bound pattern recognition receptors ( PRRs ), such as Toll-like receptors ( TLRs ).
The main thing is to process and provide antigens to T cells to stimulate their differentiation.
Dendritic cells are not only central to coordinating immune responses against threats, but are also required to regulate the immune system and induce immune tolerance in the steady state.
Specifically, dendritic cells and thymic medullary epithelial cells present self-antigens to self-reactive single positive CD4+ T cells via MHC-II and promote their apoptosis when the interaction is strengthened.
However, this process is not sufficient to completely eliminate self-reactive clones. Other mechanisms of peripheral immune tolerance help maintain immune system homeostasis and prevent responses to “self” or innocuous antigens.
Again, DCs are key to this process. Specifically, immature dendritic cells ( iDCs ) have a phenotype with lower cross-expression capacity and less expression of costimulatory molecules than mature dendritic cells ( mDCs ), which confer to them tolerance characteristics and Critical role in peripheral tolerance.
Dendritic cells can also promote peripheral tolerance of autoreactive CD8+ T cells through a mechanism called peripheral cross-tolerance, promoting clonal clearance, clonal anergy, and differentiation of regulatory T cells ( Tregs ).
Mechanism of tolerance
In humans and mice, there are several mechanisms by which tolDCs induce tolerance.
First, when T cells recognize antigens provided by tolDCs through the interaction between T cell receptors ( TCRs ) and MHC , T cells cannot generate T cells in the absence of a co-stimulatory signal ( binding of T cell CD28 to CD80/CD86 in DCs ) IL-2 and proliferate.

Second, the expression of co-repressor molecules in tolDCs propagates contact-dependent inhibitory signals in T cells, inhibits proliferation, and promotes clonal anergy.
For example, PD-L1 is an inhibitory surface receptor, and its expression by DCs is involved in tolerance induction.
Furthermore, CTLA-4 can modulate co-stimulatory molecules expressed by DCs, impairing the priming of naive T cells.

In addition to cell-cell contact-dependent mechanisms, tolDCs can produce several tolerogenic cytokines and metabolites.
Various types of tolDC produce IL-10 and can induce effector and memory CD4+ T cell anergy in vitro, as well as promote the differentiation of Treg and Breg secreting IL-10, IFN-γ and TGF-β.

Finally, tolDCs can directly eliminate T cells by clonal depletion.
For example, the interaction between dendritic cell tumor necrosis factor ( TNF )-related apoptosis-inducing ligand ( TRAIL ) and T cell death receptor can promote their apoptosis by activating the caspase pathway.
Likewise, Fas, which is upregulated during T cell activation, can bind to FasL in tolDCs and promote apoptosis in mouse T cells.

Pathways of Dendritic Cell Tolerance Generation
Immune cell differentiation, including the immunogenicity or tolerance of dendritic cells, requires precise transcriptional regulation, which mainly relies on transcription factors ( TFs ) and epigenetic mechanisms.
Different TFs have been identified in mouse cells and in in vitro human differentiation models, for example, in mice, TF PU.1 is thought to be a master regulator of cDC development.
In addition, some TFs were associated with polarization of cDC subsets: BATF3, IRF8, NFIL3, ID2, NOTCH2, IRF4 and KLF4.
TFs work together with epigenetic enzymes to target specific genomic loci, resulting in epigenetic modifications.
It is well known that epigenetic marks such as DNA methylation and histone post-translational modifications can directly or affect cellular phenotype and function, and several studies have demonstrated the role of different epigenetic mechanisms in the acquisition of DC tolerance.
For example, histone deacetylase ( HDAC ) 11 negatively regulates the expression of the IL10 gene in human and mouse dendritic cells.
Furthermore, oxidized phospholipids produced during certain inflammatory responses can differentiate human monocyte-derived dendritic cells to a tolerogenic phenotype in vitro.
Specific DNA methylation changes are also associated with acquired tolerance, and in vitro differentiation of human monocytes in the presence of prostaglandin E2 ( PGE2 ) generates a tolDC, which induces upregulation of DNMT3A, which mediates immunogenicity genes The methylation and silencing of these cells affects the ability of these cells to inhibit CD8+ T cell proliferation in vitro.
Antigen-specific DC therapy for autoimmune and inflammatory diseases
Chronic inflammatory diseases are characterized by disruption of immune homeostasis and prolonged or recurrent inflammatory responses triggered by specific endogenous or exogenous antigens.
Current treatments may involve the use of immunomodulators and immunosuppressants, ranging from small molecules such as methotrexate to monoclonal antibodies such as Ocreizumab.
Treatment of monocyte-derived tolerogenic DCs ( mo-TolDCs ) represents a promising alternative. mo-tolDCs have the potential to “re-educate” the immune system toward homeostasis by eliminating pathological autoimmune or inflammatory responses without interfering with protective immunity.
Furthermore, tolDCs may trigger a self-reinforcing tolerance circuit, making it reasonable to expect that this therapy might provide durable beneficial effects, even therapeutic ones.

So far, proof-of-concept for the potential therapeutic efficacy of tolDCs based on mouse models has been used for RA, T1D, MS, and organ transplantation.
Based on the growing understanding of tolDC biology and favorable results in animal models, there are currently multiple Phase I clinical trials evaluating the safety and feasibility of recipient-derived tolDC therapy in autoimmunity and transplantation.
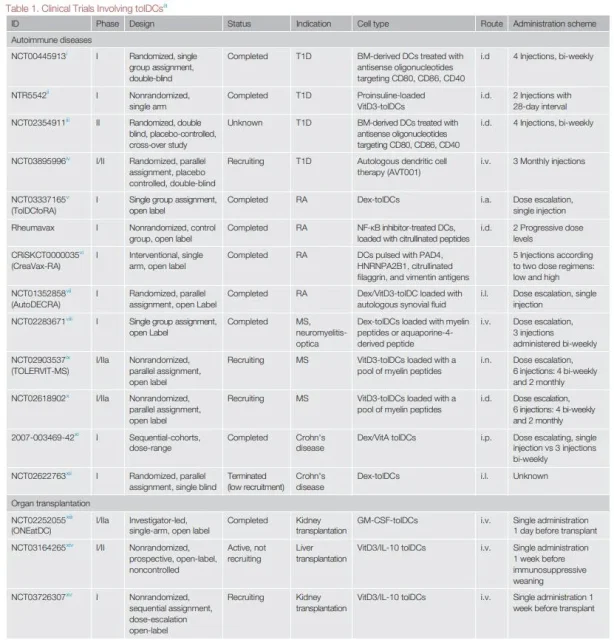
For tolDCs to be truly applied in the clinic, many problems need to be solved. Different tolDC generation protocols, doses, routes of administration, targeted diseases, and patient types involved hinder comparison of trial data and underscore the need to coordinate future clinical analyses.
Furthermore, a key consideration in developing mo-tolDC-based therapies is that monocytes isolated from patients may have different phenotypes than healthy individuals, resulting in tolDCs with different functions.
Indeed, inflammation can affect the frequency and phenotype of myeloid cells at the transcriptional and epigenetic levels.
Furthermore, the existence of numerous steps to generate tolDCs with different phenotypes and tolerances poses a great challenge, requiring the establishment of robust quality control metrics to assess the phenotype, potency and safety of each cell product.
These needs have driven the actions of agencies in the US ( Immune Tolerance Network ) and Europe ( Action to Focus and Accelerate Cell-Based Tolerance Inducing Therapies ) to coordinate trial design and immune surveillance involving tolerogenic cell products.
These synergistic actions have resulted in six Phase I/II clinical trials evaluating the use of various regulatory cell products in kidney transplantation; in addition to these, there are ongoing dose-escalation Phase I trials investigating patients with multiple sclerosis A comparative study of intradermal and transdermal administration of VitD3-tolDCs.
Outlook
In recent years , research on tolDC biology and tolerance mechanisms has advanced, and in particular, the pathways that shape these cellular tolerance programs at the transcriptomic and epigenomic levels are being better understood.
In addition, Phase I clinical trials have shown that tolDC-based therapy is safe and feasible for certain diseases.
Further studies are required to improve our understanding of the biology of tolDCs with the aim of better understanding the potential to modulate the immune properties of tolDCs in different disease contexts. In tumor-targeted cell therapy, the environment plays a key role in the success of a given treatment, suggesting the need for better control of the pathological environment in which tolDCs function.
Together with this approach, analysis of the characteristics of monocytes isolated from patients with specific diseases may play a key role in optimizing second-generation tolDC therapies.
Furthermore, for the induction of immune tolerance, epigenetic alterations and epigenetic-based treatments may provide new targets for modulating the fitness of tolDCs.
Finally, clinical trials and immune monitoring protocols must be standardized, which can help further optimize the clinical translation of tolDCs to ideally treat a variety of pathological conditions, including certain autoimmune and inflammatory diseases.
references:
1.Tolerogenic Dendritic Cells in Autoimmunity and InflammatoryDiseases. Trends Immunol. 2020 Dec 5; S1471-4906(20)30259-3.
2. Decoding the Heterogeneity of Human Dendritic Cell Subsets. TrendsImmunol. 2020 Dec;41(12):1062-1071.
Prospects of Tolerant Dendritic Cells in Autoimmune Diseases
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



