COVID-19: Multi-target collaborative small molecule antiviral drugs
- Gut Bacteria Enzymes Offer Hope for ABO Universal Blood Transfusions
- Well-Known Japanese Medicine Exposed for 30 Years of Data Falsification
- Oregon Reverses Course: From Decriminalization to Recriminalization of Drug Possession
- Why Lecanemab’s Adoption Faces an Uphill Battle in US?
- Yogurt and High LDL Cholesterol: Can You Still Enjoy It?
- WHO Releases Global Influenza Vaccine Market Study in 2024
COVID-19: Multi-target collaborative small molecule antiviral drugs
COVID-19: Multi-target collaborative small molecule antiviral drugs. In response to the new coronavirus, adhere to the development of small molecule antiviral drugs with multi-target coordination.
The number of confirmed COVID-19 worldwide is still increasing, and “specific drugs” are still not found. The winter has come, and the cold and humid weather, which is more suitable for the spread of the virus, has enveloped half of the earth. Amidst the haze of the epidemic, the “light of hope” began to emerge, and the COVID-19 vaccine and single-target small molecule drugs began to be affected by the world.
The number of confirmed COVID-19 worldwide is still increasing, and “specific drugs” are still not found. The winter has come, and the cold and humid weather, which is more suitable for the spread of the virus, has enveloped half of the earth. In the midst of the haze of the epidemic, the “light of hope” began to emerge, and the COVID-19 vaccine and single-target small molecule drugs began to be admitted to the market by governments around the world. These vaccines and small molecule drugs rely on massive amounts of capital and use the latest technology to shorten the research and development cycle that takes more than ten years to several months.
Figure: Global temperature distribution map (December 21)
On May 1, 2020, Gilead announced that the antiviral drug Remdesivir under development has obtained the U.S. FDA Emergency Use Authorization (EUA). In vitro tests have shown that it can effectively inhibit the new coronavirus and is urgently used to treat new types of coronavirus. Coronavirus pneumonia. On October 22 of the same year, the US FDA approved its antiviral drug Remdesivir (trade name: Veklury) to be officially launched. At the same time, among the more than 200 vaccines under research in the world, 13 vaccine candidates have entered phase III clinical trials, and China occupies 5 of them.
It should be noted that these vaccines and drugs have not completed the established routine drug development process. Due to the major needs of epidemic prevention and control, they have been urgently approved for use by government regulatory agencies in various countries. In fact, vaccines and drugs against the new coronavirus have not yet been applied on a large scale. They are limited by many factors. The most important issue is the lack of safety and effectiveness verification.
For example, the single targeted small-molecule drug Remdesivir approved for marketing is used in the clinical treatment of COVID-19 infections. The World Health Organization has announced the results of clinical trials of 11266 COVID-19 patients with remdesivir in 30 countries. “It has little or no effect at all in reducing the treatment of COVID-19 infection.” The results suggest that traditional single-target antiviral drugs have little effect on the treatment of COVID-19. As time progresses, clinical observations on the sequelae of infection with the new coronavirus have gradually become clearer.
At the same time, Pfizer, which was urgently approved to market the vaccine, announced that the experiment showed that the vaccine had no “obvious sequelae”, but severe allergic symptoms occurred in more than 20,000 subjects. Pfizer said there is no evidence that this is caused by the vaccine. According to statistics, after being vaccinated with Pfizer and Moderna’s COVID-19 vaccine, 2% of vaccinators will have a severe fever of 39°C to 40°C. Although this ratio is not high, if they follow their goal (35 million people inoculated within 2020), 700,000 people will have severe fever. In addition to fever symptoms, there are other possible potential side effects that need to be studied and counted.
In view of the susceptibility to drug resistance of single target drugs and the mutation and evolution of viruses, multi-target collaborative therapy provides great potential for the prevention and treatment of new coronaviruses.
The new coronavirus is an RNA virus. Compared with DNA viruses, it lacks a polymerase that can play an “error correction” and has the characteristics of rapid mutation and extremely unstable state. Therefore, from a medical point of view, the new coronavirus has mutated It is a normal phenomenon in accordance with the law and consistent with the results predicted by the Shengpu team. At present, the Furin protease cleavage site of the new coronavirus has a P681H mutation, exposing a larger open domain, which increases the binding affinity of Spike glycosphing protein to the human ACE2 receptor by 1,000 times, and the infectivity is significantly improved. At the same time, 69-70 dal of S antigen deletion occurred. This mutation is helpful for the virus to escape the host’s immune response. Faced with the rapid mutation of the new coronavirus, vaccines and single-target drugs developed in a short period of time have a relatively high probability of failure against the new coronavirus and its mutant viruses.
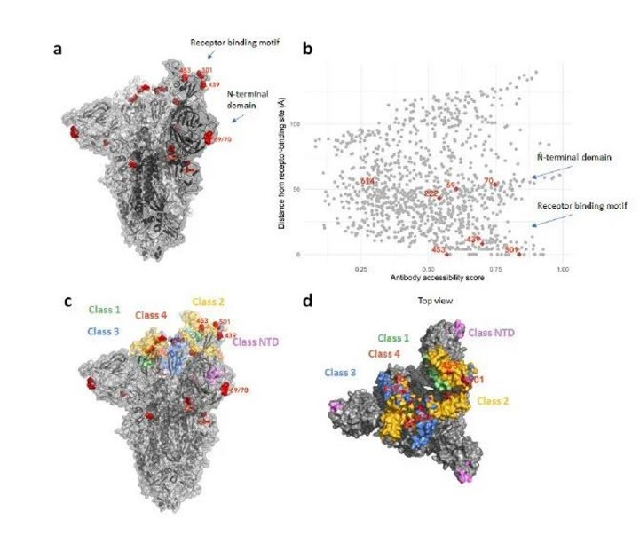
Figure: Structural variation of the new coronavirus
On December 19, British Health Minister Hancock stated at a press conference that the new strain of the new coronavirus discovered in the UK has been out of control. Hancock also said that the British health department has notified the World Health Organization of this situation and conducted research. The mutant virus may have more serious consequences or invalidate the COVID-19 vaccine and drugs. This situation is not expected to be an exception. According to the tracking research statistics of the Shengpu Health and Environmental Research Center, the new coronavirus has been confirmed to have mutated 25 times so far, with an average of two mutations per month. It is predicted that the mutation of the new coronavirus will continue, with the arrival of the winter and spring flu season, influenza viruses and COVID-19 It is possible that the virus will chimerize into a new complex virus.
In the face of complex mutations of the new coronavirus, in terms of target selection for new drug development, a single viral target cannot be selected as a therapeutic target, because drugs targeting the new coronavirus have been clinically proven to be ineffective and have side effects due to the unity of their targets . It is believed that the research and development of small molecule multi-target collaborative therapeutic drugs may be the only solution to the virus mutation. Even traceability research can be completed quickly and accurately.
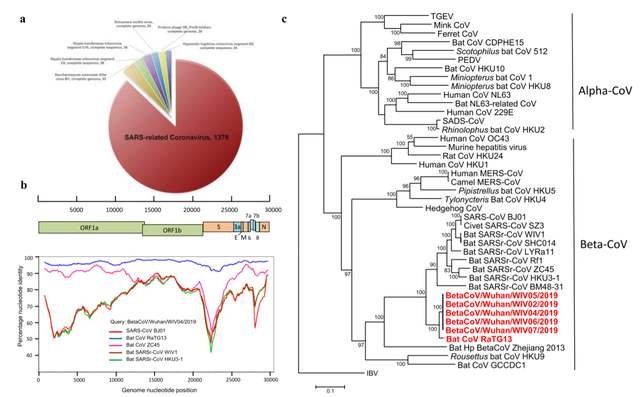
Figure: Research on the traceability of the new coronavirus
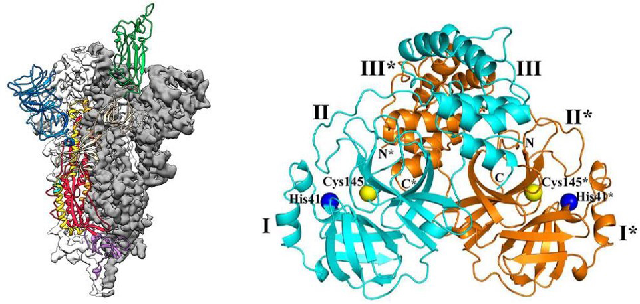
Figure 5. Structure of Spike protein and main protease Mpro
(source:internet, reference only)
Disclaimer of medicaltrend.org



