mRNA COVID-19 vaccine still effective after virus mutates
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- What is the difference between Atorvastatin and Rosuvastatin?
- How long can the patient live after heart stent surgery?
mRNA COVID-19 vaccine still effective after virus mutates
mRNA COVID-19 vaccine still effective after virus mutates. The latest research: After the virus mutates, the mRNA new coronavirus nucleic acid vaccine is effective!
Following the United Kingdom and South Africa, Japan also discovered a “new variant of the new coronavirus.”
Japan’s Ministry of Health, Labour and Welfare announced on January 10 that 4 people who had returned to Tokyo from Brazil had been diagnosed with COVID-19 pneumonia. The whole gene sequencing of the virus showed that the infecting strain was different from the British and South African mutants, or was a new type of mutant. The National Institute of Infectious Diseases of Japan stated that it is not yet clear how severe the condition will be after infection with this variant strain, or whether it will affect the efficacy of the COVID-19 vaccine.
“We have seen a large number of reports, both British and South African mutants. Genetically, we see the virus constantly mutating. This is called molecular epidemiological tracking. But in immunology, the change is not so fast. In other words, the existing vaccines can deal with the new coronavirus variants.” On January 9, Shao Yiming, consultant of the World Health Organization (WHO) Vaccine R&D Committee and researcher of the China Center for Disease Control and Prevention, told CCTV News.
Recently, the preprint platform bioRxiv issued a document stating that the mRNA COVID-19 nucleic acid vaccine BNT162b2 is still effective against mutant strains carrying N501Y mutations. Earlier reports pointed out that the variant strains in the UK and South Africa both contained the N501Y mutation.

Screenshot from bioRxiv
Sino-American Studies:Variant strain did not affect vaccine efficacy
Up to now, the World Health Organization has officially notified 4 new coronavirus variants, including the D614G variant found in Europe from January to February 2020, and the Cluster 5 variant found in Denmark from August to September 2020, December 2020 VOC variants were found in the United Kingdom in December, and N501Y.V2 variants were found in South Africa in December 2020.
Yuqi Zhao, a tenured professor at the Department of Microbiology-Immunology and Institute of Human Virology at the University of Maryland School of Medicine, told the media that it is the VOC and N501Y.V2 variants that are worrying, both of which contain the N501Y mutation. This is the 501st amino acid variation of the S protein, which is located in the RBD region of the S protein, and is the key part of the virus binding to human cell receptors. After the mutation occurs, the new coronavirus may be able to adhere to human cells more strongly, that is, the transmission power becomes stronger. Variant strains may also weaken the immune response triggered by vaccines and previous infections.
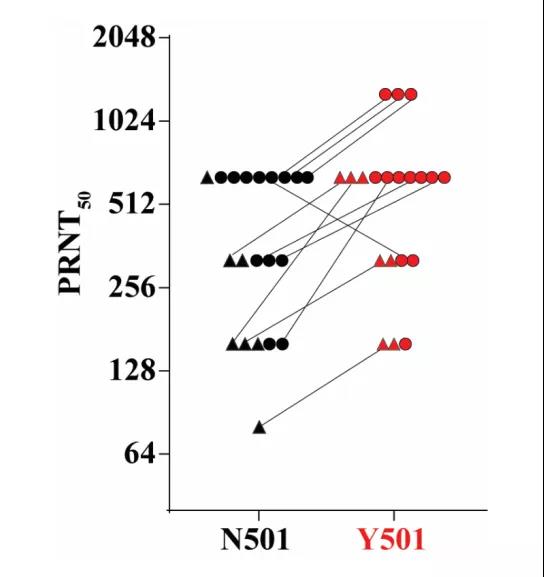
However, bioRxiv’s post is like a reassurance. The study was jointly completed by the University of Texas at Galveston Medical Branch, and its preparation was divided into two steps. First, create mutant strains. On the basis of the virus strain (N501) used to develop BNT162b2, the mutation of N501Y gene was introduced to generate a mutant strain (Y501). Second, sampling. In the clinical trial of the mRNA new coronavirus nucleic acid vaccine BNT162b2, blood samples were taken from 20 volunteers. These volunteers are all from the vaccine group and have completed two doses with an interval of 21 days. Serum sampling occurs 2-4 weeks after the second dose of vaccine.
The researchers tested the neutralizing titers of 20 serum samples against standard virus strains and variant strains (N501 and Y501). The results showed that compared with N501, the neutralizing titer of Y501 by serum samples did not decrease, but increased. The higher the titer, the stronger the neutralizing ability of the serum, that is, the higher the protective efficacy of the vaccine. As of the publication of this article, this research has not been peer reviewed.
Research in some countries has also reached similar results. At the press conference on the Joint Prevention and Control Mechanism of the State Council on January 9, Zeng Yixin, head of the vaccine research and development team of the Joint Prevention and Joint Control Mechanism of the State Council, said that two research teams in China (Institute of Medical Laboratory Animals, Chinese Academy of Medical Sciences, Sun Yat-sen University The Institute of Virology) compared the new coronaviruses that appeared in China in January, February, March, April, May, and June 2020 with the VOC variants, and found that the COVID-19 vaccine immunized monkeys and immune antibodies produced by humans. It can also neutralize the early virus strains and VOC mutant strains in China. It is said that the above two studies have been submitted and are awaiting publication. It is not clear what kind of n COVID-19 vaccine is involved.
“There is no reason to think that the vaccine is ineffective”
Researchers at the University of Texas Medical Branch at Galveston admit that there are limitations. For example, when constructing Y501 mutant strain, only one mutation site was introduced. In fact, VOC and N501Y.V2 variants have other mutations.
“Not to be underestimated.” “Science” magazine pointed out that N501Y.V2 also contains the E484 site mutation. The bioRxiv preprint platform published a joint study by the Fred Hutchinson Cancer Research Center of the United States and the University of Washington, claiming that this mutation may affect the neutralization of serum, causing the new coronavirus to be more easily combined with human cells. But the weakening of neutralization does not mean elimination. “This shows that a strong vaccine response is still protective against E484.” The research team said that the E484 mutation will not substantially impair vaccine performance. Vaccination often triggers a large number of neutralizing antibodies. When fighting against mutant strains, a slight decrease in vaccine efficacy may be less important.
The Galveston Medical Division of the University of Texas in the United States stated that “continuous monitoring” is essential, and it is necessary to evaluate the protective efficacy of the mRNA new coronavirus nucleic acid vaccine BNT162b2 against other mutations. “E484 is the next on the list to be tested.”
“China Business News” reported that in addition to effectively resisting the N501Y mutation, the mRNA COVID-19 nucleic acid vaccine BNT162b2 is also effective against 15 other mutations that have been previously tested. None of the mutations had a major impact on the effectiveness of the vaccine. “This is good news.”
Frederick Bushman, a professor of virology at the University of Pennsylvania, has been tracking virus mutations. He told ABC News: “There is no reason to believe that the mRNA COVID-19 nucleic acid vaccine will fail.”
Just 6 weeks,That can design new mRNA vaccines against mutant strains
On December 31, 2020, local time, the WHO listed the mRNA COVID-19 nucleic acid vaccine BNT162b2 as “emergency use”. This is the first new coronavirus vaccine authorized by the WHO for emergency use. As of that time, the vaccine has obtained conditional marketing, emergency use authorization or temporary authorization in more than 40 countries/regions (including the 27 EU member states).
This vaccine is called “Fosun New Coronavirus Vaccine” in China. According to previous media reports, as of December 19, Fosun Pharma has promoted the development and commercialization of the vaccine in China. Currently, the candidate vaccine BNT162b2 is undergoing phase II clinical trials in Taizhou and Lianshui, Jiangsu, China, and the recruitment of subjects Completed ahead of schedule, a total of 960 people were enrolled, and no serious adverse event reports have been received so far.
Reuters reported that the European Centers for Disease Control and Prevention recognizes the effectiveness and safety of the mRNA new coronavirus nucleic acid vaccine BNT162b2, but also particularly emphasizes the problem of mutation. The agency said that if the virus undergoes major mutations and “antigenic drift”, R&D adjustments should be made.
Frederick Bushman believes that if the mutation of the new coronavirus accumulates to a level that requires an adjustment of the vaccine, that is, the virus can evade the immune system and invalidate the antibody protection that the human body has obtained before, that is for the mRNA new coronavirus nucleic acid vaccine produced by the new technology. , It is not difficult to reorganize and design.
Short time is the outstanding advantage of mRNA technology. The mRNA novel coronavirus nucleic acid vaccine does not require time-consuming procedures such as the isolation and cultivation of mutant strains, and does not require cumbersome antigen protein expression. Once the genetic sequence of the pathogen is known, researchers can use the genetic sequence of the target virus to program the mRNA against the virus.
The National Institute of Allergy and Infectious Diseases published an article in the journal Nature Review Immunology in November 2020 that after mastering the gene sequence, an mRNA vaccine can be produced within a few weeks.
“Within 6 weeks, BioNTech will provide a new mRNA COVID-19 vaccine that completely mimics viral mutations.” On December 22, 2020, the mRNA COVID-19 vaccine BNT162b2 research and development company and the German biotech company BioNTech (BioNTech) jointly The founder Dr. Ug Sain said.
Professor Lu Hongzhou from Shanghai Public Health Clinical Center told CBN: “The mutation of the virus should be paid attention to, but people do not have to worry too much about the impact of the virus mutation on the vaccine. Because with the current vaccine technical route, it has been fully able to rely on the virus. Mutation, and redesign a vaccine against a new strain. In theory, it can be approved after completing Phase I and Phase II clinical trials, without the need to restart large-scale Phase III clinical trials.”
“Science” magazine pointed out that in the next few days, more laboratory results about the COVID-19 vaccine and variant strains are expected to be announced.
(source:internet, reference only)
Disclaimer of medicaltrend.org



