Androgen is an obstacle to longevity and castration can prolong life
- Early Biomarker for Multiple Sclerosis Development Identified Years in Advance
- Aspirin Found Ineffective in Improving Recurrence Risk or Survival Rate of Breast Cancer Patients
- Child Products from Aliexpess and Temu Contain Carcinogens 3026x Over Limit
- Daiichi Sankyo/AstraZeneca’s Enhertu Shows Positive Results in Phase III DESTINY-Breast06 Clinical Trial
- Mn007 Molecules Offer Potential for Combating Streptococcus pyogenes Infection
- Popular Indian Spices Banned in Hong Kong Over Carcinogen Concerns
Androgen is an obstacle to longevity and castration can prolong life
- AstraZeneca Admits for the First Time that its COVID Vaccine Has Blood Clot Side Effects
- Was COVID virus leaked from the Chinese WIV lab?
- HIV Cure Research: New Study Links Viral DNA Levels to Spontaneous Control
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Androgen is an obstacle to longevity and castration can prolong life
If you want to live a long life, you must first leave the palace? The latest research confirms that androgen is an obstacle to longevity, and castration can prolong life
Longevity is the eternal vision of human beings. Interestingly, among mammals, females generally live longer than males, and humans are no exception .
According to statistics from the World Health Organization and the United Nations Population Organization, the average life expectancy of men worldwide is 5-10 years shorter than that of women.
A series of recently published research results have revealed the “behind the scenes” behind the shorter life expectancy of males- androgen .
On May 23, 2023, the team of Professor James Nelson of the Institute of Longevity and Aging at the University of Texas Health Science Center at San Antonio Health Science Center published a research paper entitled: Prepubertal castration eliminates sex differences in lifespan and growth trajectories in genetically heterogeneous mice in the journal Aging Cell .
The study confirms that prepubertal castration eliminates the lifespan difference between the sexes, reduces male mortality from early to middle age, and increases the average lifespan of males to a level comparable to that of females.

Gender differences in aging and lifespan have been widely observed, and in humans, women also generally live longer than men. However, the mechanisms responsible for these differences remain poorly understood.
The study looked at the UM-HET3 mouse model, which mimics well the sex differences in human lifespan. In this mouse model, the research team explored the impact of postpubertal testicular effects on sex differences in aging.
The results show that castrating the male mice before puberty can eliminate the existing life span difference between the sexes, reduce the mortality rate of males from early to middle age, and extend the average life span of males to be on par with females. Life expectancy is comparable.
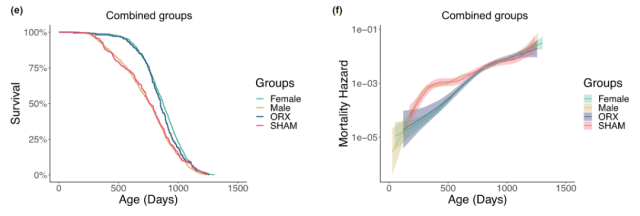
Prepubertal castration eliminates lifespan difference between sexes, ORX (castration), SHAM (sham)
In addition, the study also found that castrated male mice, despite their slower growth rate, also prolonged the duration of their weight gain, resulting in a final body weight much higher than that of normal males of the same age.
At the same time, the negative correlation between the male’s early body weight and lifespan was weakened, so that the indicators tended to be closer to those of females.
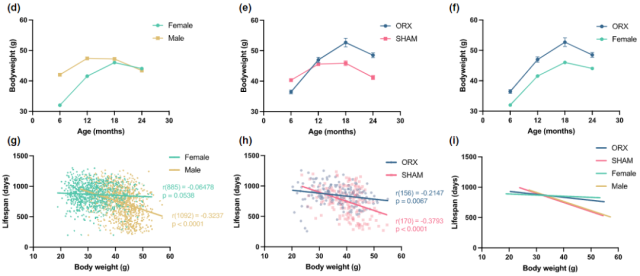
Castration shifts growth patterns and the relationship between body weight and lifespan in males to that of females
These findings suggest that postpubertal androgens are primarily responsible for sex differences in lifespan and growth trajectories. These findings provide the basis for further investigation of the underlying mechanisms driving sex-specific aging patterns and the development of potential longevity interventions.
In July 2021, a study published in the journal eLife showed that castration can affect DNA methylation modification at the epigenetic level, thereby prolonging lifespan.
The research comes from an international research team composed of the University of Otago in New Zealand and the University of California, Los Angeles. The title of the paper is: Castration delays epigenetic aging and feminizes DNA methylation at androgen-regulated loci .
The study revealed that castrated rams can delay DNA aging and live longer than normal rams.
At the same time, DNA methylation modifications in these castrated rams showed female characteristics. The research team demonstrated in sheep that castrated male mammals live longer and showed corresponding traits in their DNA.

Farmers and scientists have long known that castrated rams, on average, live much longer than non-castrated rams. In this study, for the first time, the research team looked at the reasons behind this at the DNA level.
As early as 2013, Professor Steve Horvath of the University of California, Los Angeles, combined more than 100 laboratories to organize and analyze the blood and tissue samples of more than 200 mammals and 36,000 cytosine methylation conditions, and designed a ” The epigenetic clock ,” which tracks the biological age of humans and other mammals through DNA methylation.
In this study, Professor Steve Horvath hopes to use the ” epigenetic clock ” to study the DNA differences in male mammals before and after castration, so as to reveal the reasons behind the extension of male life span by castration.
The research team used this “epigenetic clock” to observe epigenetic modifications on the DNA of castrated and normal male rams. They found that the hands of the “epigenetic clock” ticked differently, and that castrated rams tended to run more slowly.
This means that castrated rams live longer, a trait that is reflected in their DNA. In sheep, DNA aging patterns are very different in males and females. But surprisingly, the castrated rams had very feminized traits at specific DNA loci.
image
DNA of castrated rams ages more slowly
In addition, the research team also found that CpG islands on several androgen-sensitive DNA, which gradually showed a trend of hypomethylation with age, but remained stable in castrated males and normal females. Similar sex-specific methylation differences were also found in bat skin and a range of mouse tissues with high expression of the androgen receptor.
These observations suggest androgen-dependent hypomethylation in mammalian DNA, thereby providing a clear link between castration, androgenism, and sex differences in DNA aging.
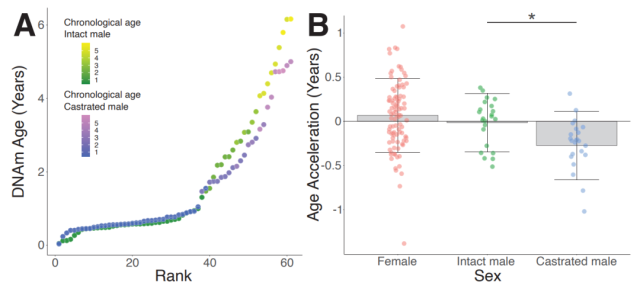
Age-associated, androgen-dependent hypomethylation in sheep DNA
To see which tissues were strongly affected by androgen levels, the research team also looked at the effects of sex in mice.
In tissues where androgen receptors are found, such as the skin, kidney and brain, there are large differences in the DNA patterns of males and females. In contrast, tissue without androgen receptor expression looked the same in males and females.
Collectively, these studies have shown that androgens are key factors leading to sex differences in lifespan.
Determining the mechanisms behind these sex differences is crucial for the development of corresponding life-extending targets. The harmful effects of the target without affecting the male function will undoubtedly become a major breakthrough in the field of anti-aging and life extension.
Paper link :
1. https://onlinelibrary.wiley.com/doi/10.1111/acel.13891
2. https://elifesciences.org/articles/64932
Androgen is an obstacle to longevity and castration can prolong life
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.