Unlocking Immunogenicity in mRNA Vaccines: Analysis and Insights
- EB Virus Could Be Infected by Kiss: A Hidden Threat Linked to Cancer
- The Silent Threat: How Gas Stoves Pollute Our Homes and Impact Health
- Paternal Microbiome Perturbations Impact Offspring Fitness
- New Report Casts Doubt on Maradona’s Cause of Death and Rocks Manslaughter Case
- Chinese academician unable to provide the exact source of liver transplants
- Early Biomarker for Multiple Sclerosis Development Identified Years in Advance
Unlocking Immunogenicity in mRNA Vaccines: Analysis and Insights
- AstraZeneca Admits for the First Time that its COVID Vaccine Has Blood Clot Side Effects
- Was COVID virus leaked from the Chinese WIV lab?
- HIV Cure Research: New Study Links Viral DNA Levels to Spontaneous Control
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Unlocking Immunogenicity in mRNA Vaccines: Analysis and Insights
On October 2, the highly anticipated 2023 Nobel Prize in Physiology or Medicine was awarded to Hungarian scientist Katalin Karikó and American scientist Drew Weissman.
This recognition was for their groundbreaking discoveries in nucleoside modification, which paved the way for the development of effective mRNA vaccines against COVID-19.
Let’s delve into the hot topic of mRNA vaccines and discuss their immunogenicity analysis.
Background
At the beginning of 2020, the COVID-19 virus outbreak prompted an urgent need to develop safe and effective vaccines. In the race to combat the pandemic, mRNA vaccines gained prominence due to their short development timelines and relatively straightforward production.
Several mRNA vaccines have now been approved for use worldwide, including BioNTech and Pfizer’s BNT162b2 (sold as COMIRNATY), Moderna’s mRNA-1273 (SPIKEVAX), and Sinopharm Group’s SYS6006, among others.
Beyond COVID-19 protection, mRNA vaccines are being explored for various applications, such as Pfizer’s endeavors in infectious diseases and Moderna’s promising clinical results in cancer and infectious disease research.
Several mRNA vaccine research companies are also focusing on infectious diseases and oncology. This article delves into the central aspect of mRNA vaccine mechanisms: immunogenicity and its biological analysis.
Mechanism of Action
The core mechanism of mRNA vaccines involves introducing mRNA sequences into host cell cytoplasm, where the host cells translate the mRNA into protein antigens. This process ultimately triggers an antigen-specific immune response in the body, leading to the production of antigen-specific antibodies and the formation of immune memory. In the process of antigen-specific immune responses, B lymphocytes are specifically activated, leading to the production of antigen-specific antibodies, mediating humoral immune responses. Helper T cells (CD4+) and cytotoxic T cells (CD8+) are also activated, secreting cytokines, activating B lymphocytes, and exerting cytotoxic effects, mediating cellular immune responses. Compared to traditional inactivated vaccines or subunit vaccines, mRNA-encoded antigens can be produced more persistently within the body, leading to a more sustained and robust immune response, providing significant immunoprotection advantages.
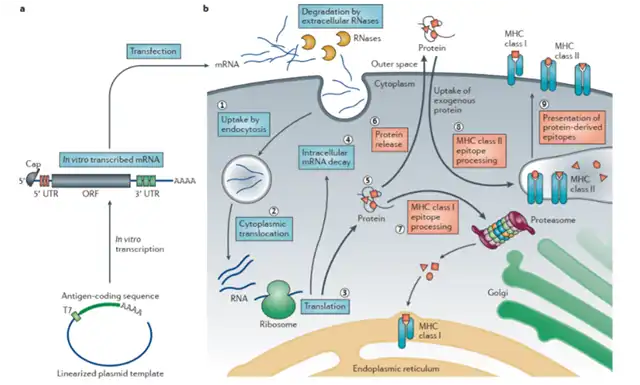
Figure 1 Mechanism of action of mRNA vaccine
Delivery Methods
mRNA molecules have relatively large molecular weights, are hydrophilic, and have a relatively simple industrial production process.
However, mRNA itself is not very stable and carries a negative charge. Thus, ensuring its stable presence within the body and successful delivery into the cell cytoplasm is crucial for mRNA vaccine development.
To prevent mRNA from degradation before entering the cell cytoplasm and its subsequent degradation within cytoplasmic lysosomes, lipid nanoparticles (LNPs) are predominantly used as carriers for mRNA delivery. LNPs consist mainly of cationic lipids, helper lipids, cholesterol, and PEGylated lipids.
LNPs encapsulate mRNA, enhancing its stability, and can fuse with the cell membrane to facilitate mRNA internalization into endosomes, where mRNA is released into the cell cytoplasm.
With the market introduction of mRNA-LNP vaccines developed by companies like BioNTech, LNP delivery technology has entered a period of rapid development.
Although a few companies, such as Arbutus, currently hold patents for LNP technology, as more manufacturers invest in LNP research, it is expected that technological barriers will be overcome, unleashing the full potential of mRNA-LNP vaccines.
Introduction to the Immunogenicity of mRNA Vaccines
mRNA vaccines typically consist of mRNA wrapped in LNPs, and their immunogenicity arises from three main components: the mRNA sequence itself, the LNP delivery vehicle, and the mRNA translation products.
Exogenous mRNA possesses some inherent immunogenicity concerns and can trigger innate immune responses through Toll-like receptors (TLRs), resulting in the production of inflammatory cytokines like TNF-α and IFN-γ. By optimizing mRNA sequences, using human codons, optimizing untranslated regions (UTRs), introducing 5′ cap structures, and 3′ poly-A tail sequences, the immunogenicity of mRNA can be significantly reduced.
As previously mentioned, LNPs primarily consist of cationic lipids, helper lipids, cholesterol, and PEGylated lipids. Lipids pose a relatively low risk of inducing an immune response, but the presence of PEG can lead to the development of anti-PEG antibodies in the body, thus accelerating the rapid clearance of carriers within the body. This can alter the in vivo distribution and bioavailability of mRNA vaccines, affecting product safety and efficacy. If patients have pre-existing high levels of anti-PEG antibodies, the administration of PEG-modified carriers may lead to life-threatening allergic reactions. Therefore, measuring the levels of anti-PEG antibodies in patients can aid in assessing the safety and efficacy of mRNA vaccines and effectively controlling post-administration allergic reactions.
Once mRNA enters the cell cytoplasm, it is expressed as a protein antigen. Protein antigens are highly immunogenic and activate both humoral and cellular immune responses. In terms of humoral immune responses, this involves B lymphocyte activation, leading to the production of antigen-specific antibodies (IgG) that prevent infectious diseases by binding to antigens. Regarding cellular immune responses, CD4+/CD8+ T lymphocytes are activated, leading to the secretion of cytokines, activation of B lymphocytes, and the exertion of cytotoxic effects, aiding in cellular immune responses. The strength of mRNA-expressed antigen immunogenicity, the level of antibody production it mediates, and the activation level of antigen-specific T cells all determine the effectiveness of mRNA vaccines, which is a critical area of clinical analysis.
Regulations and Guidelines for Immunogenicity Analysis of mRNA Vaccines
Relevant guidance from the Center for Drug Evaluation (CDE) of the National Medical Products Administration (NMPA) includes:
- “Technical Guidelines for Clinical Trials of Active Immune Oncology Products (Trial),” 2023.
- “Technical Points for Non-clinical Evaluation of Novel Coronavirus Preventive Vaccines (Trial),” 2020.
- “Technical Guidelines for Drug Immunogenicity Research,” 2021.
- “Guidance for Industry – Clinical Considerations for Therapeutic Cancer Vaccines,” 2011.
- “Guidance for Industry – Development and Licensure of Vaccines to Prevent COVID-19,” 2020.
Immunogenicity Analysis Strategies for mRNA Vaccines
In registered clinical trials, the immunogenicity analysis of mRNA vaccines primarily focuses on the detection of antibody responses to antigens and the assessment of cellular immune responses.
Antibody response testing mainly involves the detection of antigen-specific binding antibodies and neutralizing antibodies (NAbs) in serum samples, using methods such as highly sensitive electrochemiluminescence (MSD) or enzyme-linked immunosorbent assay (ELISA).
The MSD method operates by forming a “antigen peptide – antibody (IgG) – detection antibody” complex by binding antigen peptides to a 96-well plate, followed by the addition of serum samples and a detection antibody (e.g., ruthenium-labeled anti-IgG antibody) to reaction wells. After adding the substrate, the antigen-specific antibody levels in the serum samples are assessed based on chemiluminescent signal values.
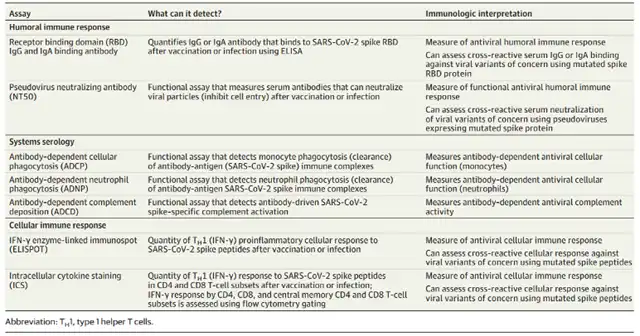
Table 1 Immunological analysis of COVID-19 mRNA vaccine
Combining Antibody (IgG) Detection Strategies:
For the detection of antigen-specific binding antibodies, ultra-sensitive electrochemiluminescence (MSD) or enzyme-linked immunosorbent assay (ELISA) methods can be employed. The principle of the MSD method is illustrated in Figure 2. Antigen peptides are coated on a 96-well plate, followed by the addition of serum samples and a detection antibody (e.g., Ru-labeled anti-IgG antibody) into the wells, forming an “antigen peptide – antibody (IgG) – detection antibody” complex. After the addition of a substrate to the wells, the level of antigen-specific antibodies in the serum samples is assessed by measuring the chemiluminescent signal.
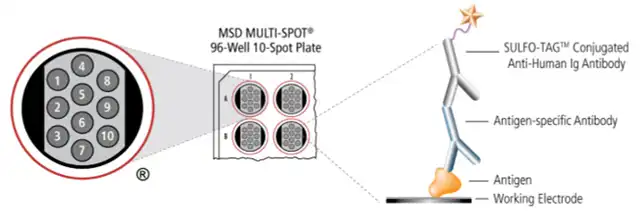 Figure 2: Schematic of the MSD Method
Figure 2: Schematic of the MSD Method
Based on the MSD method, the analysis of the levels of binding antibodies induced by mRNA-1273 and BNT-162b2-mediated immunity (as shown in Figure 3A) reveals that after a single dose of mRNA vaccine, individuals produce binding antibody levels comparable to those in convalescent COVID-19 patients and significantly higher than uninfected individuals. Furthermore, after a double dose of mRNA vaccine, individuals produce binding antibody levels far higher than those in convalescent patients. mRNA-1273 and BNT-162b2 both exhibit high immunogenicity and effectiveness in the body, with mRNA-1273 showing greater immunostimulatory strength.
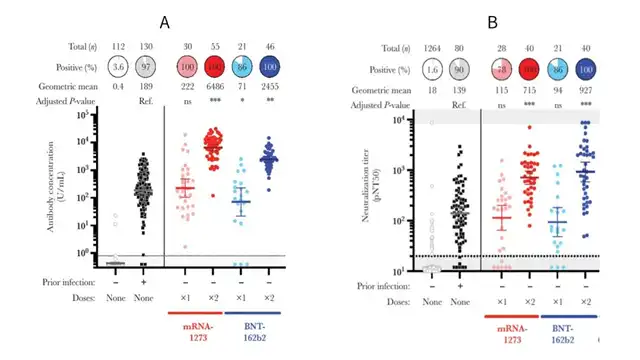
Figure 3: Immunogenicity of mRNA-1273 and BNT-162b2
A. Binding Antibody Assay B. Neutralizing Antibody Titer Assay
Neutralizing Antibody (NAb) Detection Strategies:
For mRNA vaccines, especially prophylactic vaccines like the COVID-19 mRNA vaccines, testing for humoral immune responses also involves the detection of NAbs. NAbs induced by COVID-19 mRNA vaccines can specifically bind to the spike protein of the virus, inhibit its binding to the ACE2 receptor on cell surfaces, and thus protect the body from infection by the virus.
NAb titers are important indicators for assessing the immunogenicity and effectiveness of COVID-19 mRNA vaccines. For COVID-19 mRNA vaccines, NAb detection methods mainly involve pseudovirus assays (as seen in Figure 4).
Pseudoviruses, constructed using lentiviruses or retroviruses as vectors, express the spike protein of the novel coronavirus and are packaged with luciferase and/or GFP reporter genes. These pseudoviruses can efficiently infect cells that overexpress ACE2 (e.g., 293T-ACE2), leading to the expression of luciferase.
The level of luciferase activity is positively correlated with the number of infected cells. In the NAb detection assay, serum samples are serially diluted and incubated with pseudovirus and cells.
Neutralizing antibodies present in the serum specifically bind to the pseudovirus, inhibiting transduction of the cells by the pseudovirus, resulting in reduced luciferase expression and a decrease in fluorescence signal. Analyzing the degree of reduction in the fluorescence signal allows for the assessment of NAb titers in serum samples.
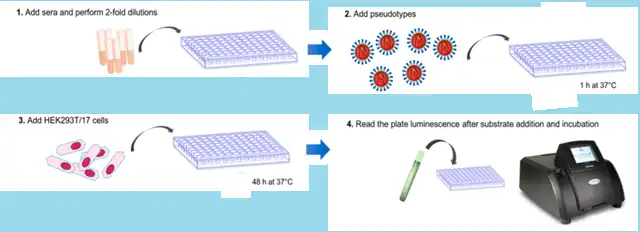 Figure 4: Pseudovirus Neutralization Assay
Figure 4: Pseudovirus Neutralization Assay
Based on pseudovirus neutralization assays, the results for the NAb titers induced by mRNA-1273 and BNT162b vaccines are shown in Figure 3B. An assay threshold value (pNT50) of 20 was established using samples from unvaccinated convalescent individuals. Under this threshold condition, 90.1% of unvaccinated convalescent individuals tested positive for NAbs. Following a single dose of mRNA-1273 and BNT162b, individuals displayed geometric mean titers equivalent to convalescent patients. After two doses of mRNA-1273 and BNT162b, all vaccinated individuals tested positive for NAbs, with significantly higher geometric mean titers, demonstrating robust effectiveness.
Cellular Immune Response Detection Strategies:
In addition to activating B cells to produce antibodies, mRNA vaccines can activate T cells, mediating cellular immune responses. Activated antigen-specific T cells can be maintained at higher levels in the body and exhibit high efficiency in killing tumor cells. Currently, cellular immune responses induced by mRNA vaccines are typically tested using assays such as enzyme-linked immunospot assays (ELISpot).
ELISpot is a sensitive method used in cellular immunology research to detect cytokine-secreting cells or antibody-secreting cells at the single-cell level, thereby assessing the strength of cellular immune responses. The principle of the ELISpot assay is illustrated in Figure 5. Antigens or antigen peptide libraries are mixed with peripheral blood mononuclear cells (PBMCs) in reaction wells. Lymphocyte T cells locally produce cytokines (e.g., IFN-γ) after being stimulated by the antigen, which are captured by antibodies immobilized on the bottom of the ELISpot plate. After removing the cells, detection antibodies, enzyme conjugates, and substrate are sequentially added to the wells, resulting in the formation of multiple spots on a PVDF membrane on the plate. The greater the number of spots, the more cells are secreting that particular cytokine. Analyzing the number of spots allows for the assessment of the number of antigen-specific T cells and the strength of the T cell immune response induced in the body.
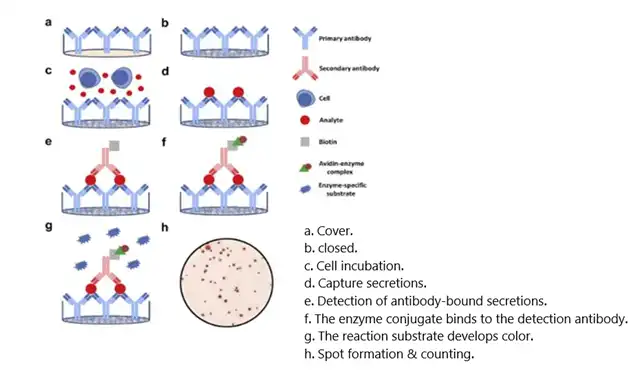 Figure 5: ELISpot Assay Principle
Figure 5: ELISpot Assay Principle
Biological functionality of PBMCs is crucial for the accuracy of the ELISpot assay results. Various factors, such as the time from whole blood extraction to PBMC separation, extraction methods, storage conditions, transportation, and thawing and recovery steps, may affect the activity of PBMCs and subsequently impact the evaluation of assay results.
Therefore, standardization of PBMC handling is required before conducting ELISpot assays to ensure the preservation of adequate extraction yields while maintaining their biological activity. Another important consideration for T cell immune response detection in ELISpot assays is the selection of antigens or peptide sequences.
The choice of antigens/peptides determines whether antigen-specific T cells can be detected in the ELISpot assay. Selecting protein antigens, considering their uptake and presentation by antigen-presenting cells (APCs) in the body, allows for the detection of all antigen-specific CD4+ T cells.
Choosing peptide libraries, given their shorter peptide sequences and HLA compatibility, can be used to detect antigen peptide-specific CD8+ T cells and/or CD4+ T cells.
Currently, for mRNA vaccine-like drugs, synthetic peptide libraries are typically selected for ELISpot assays to detect T cell immune responses, and the composition and quality of these peptide libraries need to be closely monitored.
Researchers conducted IFN-γ ELISpot assays using a SARS-CoV-2 spike protein peptide library to detect T cell immune responses induced by mRNA-1273, BNT162b, and Ad26.COV2.S vaccines. The assay results are shown in Figure 6, with a threshold value of 10 SFU/106 PBMCs. Almost all uninfected and unvaccinated individuals tested negative for PBMCs in the assay, while most individuals vaccinated with mRNA-1273 and BNT162b exhibited high spot counts in the assay (with average values of 511 SFU/106 PBMCs and 348 SFU/106 PBMCs, respectively), indicating a positive response to the vaccine and a robust antigen-specific T cell immune response. Both mRNA-1273 and BNT162b demonstrated good effectiveness.
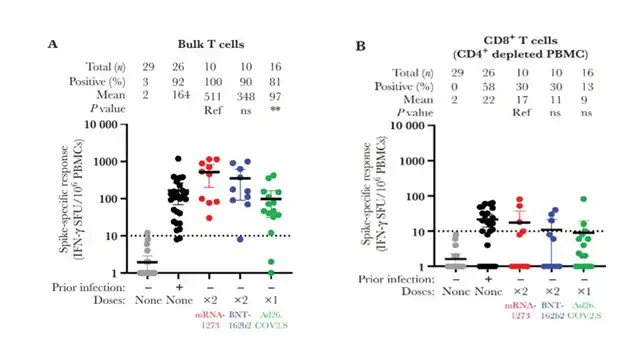 Figure 6: Cellular Immune Response Detection of mRNA-1273 and BNT-162b2
Figure 6: Cellular Immune Response Detection of mRNA-1273 and BNT-162b2
A. T Cell Immune Response Assay B. CD8+ T Cell Immune Response Assay
In Conclusion:
mRNA vaccines represent a significant breakthrough in vaccine technology and have played a pivotal role in the fight against the COVID-19 pandemic. Their immunogenicity analysis is a critical component of clinical development, as it determines the effectiveness and safety of these vaccines.
Immunogenicity assessment for mRNA vaccines includes the analysis of antigen-specific antibody responses, neutralizing antibody levels, and cellular immune responses. Techniques such as ELISA, MSD, virus neutralization assays, and ELISPOT assays are essential tools for evaluating vaccine performance.
As the field of mRNA vaccine research expands to include applications beyond infectious diseases, such as cancer and other chronic conditions, a deep understanding of immunogenicity will continue to be crucial for developing safe and effective vaccines.
It is worth noting that the provided information is based on publicly available data up to my knowledge cutoff date in January 2022, and the field of mRNA vaccine research may have evolved since that time. For the latest developments and regulatory updates, it is recommended to consult relevant health authorities and scientific literature.
References:
-
World Health Organization (WHO). (2021). mRNA Vaccines Against COVID-19: Pfizer-BioNTech COVID-19 Vaccine (BNT162, PF-07302048). Technical Report.
-
World Health Organization (WHO). (2021). mRNA Vaccines Against COVID-19: Moderna COVID-19 Vaccine (mRNA-1273). Technical Report.
-
Moderna. (2023). Clinical Studies.
-
Arbutus. (2022). Lipid Nanoparticle (LNP) Programs.
-
European Medicines Agency (EMA). (2020). EMA Regulatory Science to 2025: Strategic Reflection.
-
Center for Drug Evaluation (CDE) of the National Medical Products Administration (NMPA). (2023). Technical Guidelines for Clinical Trials of Active Immune Oncology Products (Trial).
-
Center for Drug Evaluation (CDE) of the National Medical Products Administration (NMPA). (2020). Technical Points for Non-clinical Evaluation of Novel Coronavirus Preventive Vaccines (Trial).
-
Center for Drug Evaluation (CDE) of the National Medical Products Administration (NMPA). (2021). Technical Guidelines for Drug Immunogenicity Research.
-
U.S. Food and Drug Administration (FDA). (2011). Guidance for Industry – Clinical Considerations for Therapeutic Cancer Vaccines.
-
U.S. Food and Drug Administration (FDA). (2020). Guidance for Industry – Development and Licensure of Vaccines to Prevent COVID-19.
-
Teijaro, J. R. et al. (2020). Pathogenicity and host responses of SARS-CoV-2 Delta variant in mice. The Journal of Experimental Medicine, 218(9), e2021942. doi:10.1084/jem.2021942
-
Roche Diagnostics. (2023). Mesoscale Discovery (MSD).
Unlocking Immunogenicity in mRNA Vaccines: Analysis and Insights
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



