DLBCL: Lenzilumab combined with CAR-T cell therapy 100% effective
- Oregon Reverses Course: From Decriminalization to Recriminalization of Drug Possession
- Why Lecanemab’s Adoption Faces an Uphill Battle in US?
- Yogurt and High LDL Cholesterol: Can You Still Enjoy It?
- WHO Releases Global Influenza Vaccine Market Study in 2024
- HIV Infections Linked to Unlicensed Spa’s Vampire Facial Treatments
- A Single US$2.15-Million Injection to Block 90% of Cancer Cell Formation
Diffuse large B-cell lymphoma (DLBCL): Lenzilumab plus CAR-T cell therapy 100% effective
DLBCL: Lenzilumab combined with CAR-T cell therapy 100% effective. Anti-GM-CSF monoclonal antibody lenzilumab combined with Yescarta in the treatment of Diffuse large B-cell lymphoma (DLBCL): the effective rate is 100%, no serious CRS/NT events!
Humanigen is a clinical-stage biopharmaceutical company focusing on the prevention and treatment of excessive immune responses called “Cytokine Storm” through its lead drug candidate anti-human GM-CSF monoclonal antibody lenzilumab.
Recently, the company announced the positive data of the phase 1b part of the ZUMA-19 study, which is evaluating the efficacy and safety of lenzilumab combined with CAR-T cell therapy in the treatment of patients with diffuse large B-cell lymphoma (DLBCL).
The results showed that under the recommended phase 2 dose of lenzilumab, the objective response rate (ORR) reached 100%, and no patients had severe cytokine release syndrome (CRS) or severe neurotoxicity (NT).
ZUMA-19 is a clinical study that is evaluating the efficacy and safety of lenzilumab and Gilead CD19 CAR-T cell therapy Yescarta (axicabtagene ciloleucel, Axi-Cel) in the treatment of relapsed or refractory DLBCL.
The study used a standard 3+3 design. Three patients received 600 mg lenzilumab before CAR-T (cohort 1), and 3 patients received 1800 mg lenzilumab before CAR-T (cohort 2). The recommended phase 2 dose was determined to be 1800 mg.
Among the 6 study patients, the ORR was 83% (n=5), including 4 complete remissions (CR). In cohort 1, no severe CRS (≥ grade 3) occurred.
One patient experienced grade 3 NT for 2 days. In the recommended phase 2 dose of lenzilumab (cohort 2), the ORR was 100% (n=3), and the non-toxic CR (CRS and NT<2) was 66% (n=2).
At the recommended Phase 2 dose, no severe CRS or severe NT occurred. Throughout the study, no adverse events were attributed to lenzilumab.
Inflammation markers are related to the reduction rate of CRS and NT. Lenzilumab dose-dependently reduced myeloid cytokines IL-6, IL-8, MCP-1 and IP-10 (CXCL-10) and systemic inflammation markers CRP, ferritin and SAA.
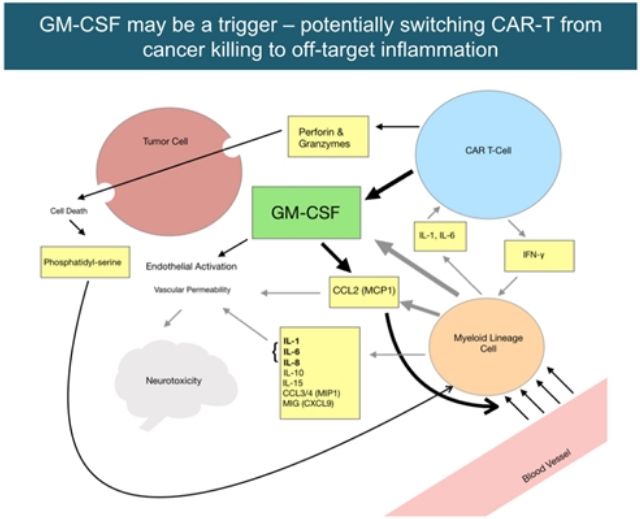
The scientific basis of GM-CSF as the initiating factor of CAR-T related toxicity
Humanigen Chief Scientific Officer Dale Chappell, MD, said: “These encouraging results from the ZUMA-19 study further prove that lenzilumab may break the link between the efficacy and toxicity (CRS and NT) that are broadly related to CAR-T, and May improve the durability of remission. We believe these data prove the need for a larger study involving multiple CAR-T cell therapies.”
Humanigen will initiate a randomized, multicenter, potential registration Phase 2 study to evaluate the efficacy and safety of lenzilumab in combination with all commercially available CD19 CAR-T cell therapies in the treatment of DLBCL patients. This research plan enrolls about 150 patients, and the research plan is being submitted to the FDA.
Humanigen has terminated the ZUMA-19-related clinical cooperation agreement signed with Kite, a cell therapy company under Gilead, and the two parties will cooperate to end current research activities.
Cameron Durrant, CEO of Humanigen, said: “Humanigen is very pleased to be able to actively develop lenzilumab throughout the CAR-T field and further expand its product line. We thank Kite for sponsoring and contributing so that Humanigen can develop to this exciting point. .”

Lenzilumab is an anti-human granulocyte macrophage colony stimulating factor (GM-CSF) neutralizing antibody. GM-CSF is a cytokine that plays a key role in acute and chronic inflammation of tissues. Lenzilumab can target to bind to and neutralize the activity of GM-CSF, inhibit the inflammatory cascade initiated by GM-CSF, thereby preventing and treating the cytokine storm and neurotoxicity associated with GM-CSF during CAR-T treatment.
In August 2017, Roche’s anti-inflammatory drug IL-6R antibody Actemra (Amerol, generic name: tocilizumab, tocilizumab) was approved by the U.S. FDA for the treatment of patients aged 2 years and older for the treatment of severe or Life-threatening CRS. This approval makes Actemra the first treatment for CRS.

CAR-T Therapy-Black Box Warning
Yescarta is a CD19 CAR-T cell therapy, which was acquired by Geely Dehao to acquire Kite for US$11.9 billion. In the United States, Yescarta was approved by the FDA in October 2017. It is the first CAR-T cell therapy for adult patients with relapsed or refractory large B-cell lymphoma (LBLC). The specific indications are:
For the treatment of adult patients with relapsed or refractory LBCL who have previously received 2 or more systemic therapies, including diffuse large B-cell lymphoma (DLBCL),
- Primary mediastinal large B-cell lymphoma (PMBCL),
- High-grade B-cell lymphoma (HGBL),
- DLBCL (ie transforming FL, TFL) derived from follicular lymphoma (FL).
Yescarta is not suitable for the treatment of primary central nervous system lymphoma.
In March 2021, Yescarta was approved by the US FDA for a new indication: for the treatment of relapsed or refractory (R/R) follicular lymphoma (FL) that has previously received 2 or more systemic therapies. Adult patients.
It is worth noting that the currently marketed CAR-T cell therapies (including Yescarta) have black box warnings about CRS and NT in their US prescription information.
(source:internet, reference only)
Disclaimer of medicaltrend.org



