Bristol-Myers’ cardiac myosin allosteric inhibitor mavacamten Phase 3 trials
- A Single US$2.15-Million Injection to Block 90% of Cancer Cell Formation
- WIV: Prevention of New Disease X and Investigation of the Origin of COVID-19
- Why Botulinum Toxin Reigns as One of the Deadliest Poisons?
- FDA Approves Pfizer’s One-Time Gene Therapy for Hemophilia B: $3.5 Million per Dose
- Aspirin: Study Finds Greater Benefits for These Colorectal Cancer Patients
- Cancer Can Occur Without Genetic Mutations?
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- What is the difference between Atorvastatin and Rosuvastatin?
- How long can the patient live after heart stent surgery?
Bristol-Myers’ cardiac myosin allosteric inhibitor mavacamten Phase 3 trials
Bristol-Myers’ cardiac myosin allosteric inhibitor mavacamten Phase 3 trials. Hypertrophic cardiomyopathy (HCM) innovative drug! Bristol-Myers’ cardiac myosin allosteric inhibitor mavacamten Phase 3 clinical trial: continuous improvement of health status!
Bristol-Myers Squibb (BMS) recently announced the new analysis of mavacamten in the treatment of obstructive hypertrophic cardiomyopathy (oHCM) Phase 3 EXPLORER-HCM study (NCT03470545) at the 70th Annual Scientific Meeting of the American College of Cardiology (ACC.21) Data: At 30 weeks of treatment, compared with the placebo group, patients in the mavacamten group showed continuous improvement in their health.
This analysis confirmed the benefits of mavacamten on the health of oHCM patients. Relevant data has also been published in the international medical journal “The Lancet” (The Lancet), the article title is: Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): health status analysis of a randomised, double-blind, placebo -controlled, phase 3 trial.
mavacamten is a first-in-class, oral, cardiac myosin inhibitor developed for the treatment of symptomatic oHCM patients. oHCM is a chronic heart disease with a high incidence, and mavacamten can solve the inherent molecular defects of the disease. Currently, mavacamten is under review by the US FDA, and the target date of the Prescription Drug User Fee Act (PDUFA) is January 28, 2022. In July 2020, the FDA granted mavacamten Breakthrough Drug Designation (BTD) for oHCM.
EXPLORER-HCM is a double-blind, placebo-controlled Phase 3 study in symptomatic oHCM patients (LVOT gradient ≥50mmHg, NYHA II-III). In the study, patients were randomly assigned to mavacamten (n=123) or placebo (n=128) at a 1:1 ratio, and were treated for 30 consecutive weeks, followed by an 8-week washout period. Previously published results showed that mavacamten showed a strong therapeutic effect, with clinically significant improvements in symptoms, functional status and quality of life, and showed the ability to relieve left ventricular outflow tract obstruction. In the EXPLORER-HCM study, all primary and secondary endpoints reached statistical significance.
The latest analysis released this time involves the Kansas City Cardiomyopathy Questionnaire (KCCQ; range 0-100 points, higher scores indicate better health), which is a disease-specific questionnaire that includes 23 items and is useful for patients’ daily lives. All aspects are quantified, including symptoms, physical function, social function, and quality of life. The KCCQ survey was carried out at the baseline and at 6, 12, 18, 30, and 38 weeks. The mixed repeated measures model (MMRM) and responder analysis method were used to analyze the changes in KCCQ scores from baseline. A total of 92 patients in the mavacamten group and 88 patients in the placebo group completed the baseline examination and the 30th week (end of treatment) KCCQ questionnaire at the same time.
The analysis results showed:
(1) At the 30th week of treatment, the change of KCCQ summary score (KCCQ-OSS) in the mavacamten group was significantly greater than that in the placebo group (mean ± SD: 14.9 ± 16 vs 5.4 ± 14; difference = 9.1 [95% CI: 5.5-12.8]; p<0.001), the benefits are similar in all KCCQ subscales.
(2) Compared with the placebo group, a higher proportion of patients in the mavacamten group achieved a very large and clinically significant improvement (KCCQ-OSS change ≥20 points: 36%[33/92] vs 15%[13/ 88]), changes of ≥5 points are considered clinically significant, and these benefits return to baseline levels after stopping treatment.
(3) A higher proportion of patients in the placebo group had worsening or no change in their health at the 30th week.
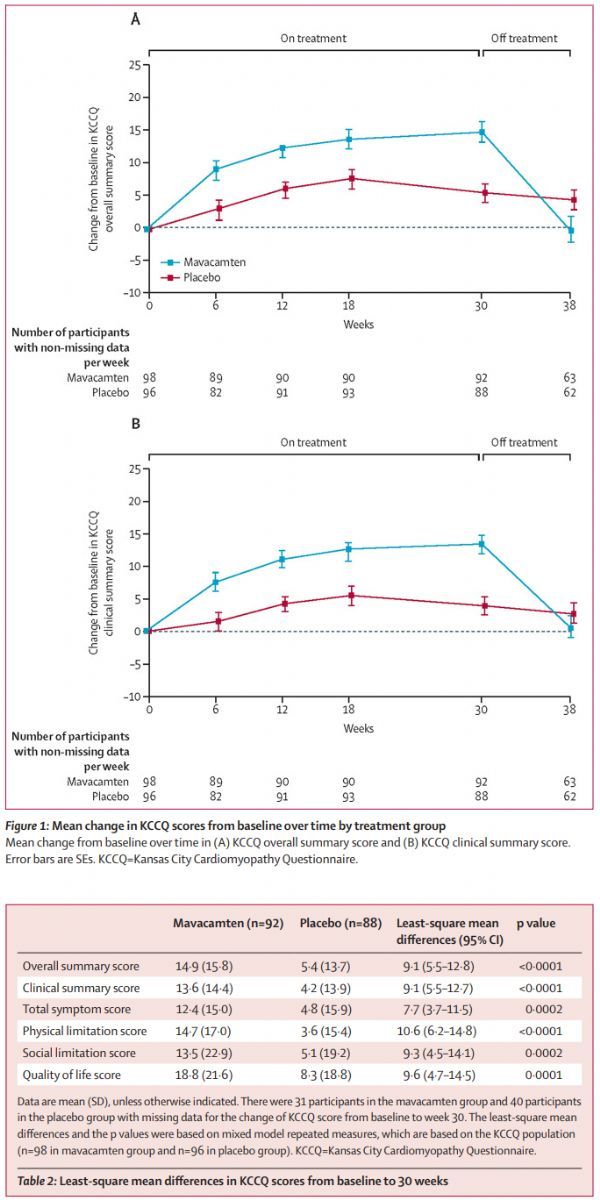
EXPLORER-HCM research new analysis data (click the picture to see the big picture)
John A. Spertus, the lead investigator of the EXPLORER-HCM study and a professor of medicine at the University of Missouri-Kansas City, said: “KCCQ is a 23 disease-specific questionnaire that quantifies symptoms, physical function, social function, and quality of life. Through Using this tool, we were able to prove that patients taking mavacamten in the trial have substantial clinical benefits, which will diminish when the patients finish treatment. This new analysis of data from the EXPLORER-HCM study is myos Protein inhibition provides important insights in improving the health of patients with severe obstructive hypertrophic cardiomyopathy (oHCM, a chronic and often debilitating disease).”
Jay Edelberg, MD, Head of Heart Failure and Cardiomyopathy Development at Bristol-Myers Squibb said: “Mavacamten represents Bristol-Myers Squibb’s continuous commitment to improve the lives of patients through scientific discovery, especially those suffering from chronic cardiovascular diseases such as oHCM. This new analysis of data from the Phase 3 EXPLORER-HCM study further supports scientific evidence that mavacamten is beneficial in improving the health, symptoms and quality of life of patients with symptomatic oHCM. We look forward to the possibility of this important issue next year. Bring new treatments to patients.”
Hypertrophic cardiomyopathy (HCM) is the most common single-gene inherited heart disease. It is a chronic, debilitating, and progressive disease. Patients may experience symptoms of shortness of breath, dizziness and fatigue, as well as severe, Life-changing complications, including heart failure, arrhythmia, stroke, and sudden cardiac death.
mavacamten is a first-in-class, oral, allosteric myosin inhibitor for the treatment of diseases that are inherently caused by excessive heart contraction and impaired diastolic filling. Mavacamten is thought to reduce the myocardial contractility by inhibiting the formation of excessive myosin-actin cross-bridges. The formation of excessive myosin-actin cross bridge can lead to excessive myocardial contraction, left ventricular hypertrophy and reduced compliance. In clinical and preclinical studies, mavacamten continues to show biomarkers that reduce heart wall stress, reduce excessive myocardial contraction, and increase diastolic compliance.
In July 2020, the US FDA granted mavacamten a breakthrough drug designation for oHCM. mavacamten was originally developed to treat symptomatic obstructive hypertrophic cardiomyopathy (oHCM). Based on its mechanism of action and evidence of therapeutic activity, mavacamten is also being clinically studied for the treatment of symptomatic non-obstructive hypertrophic cardiomyopathy (HCM) and heart failure with preserved ejection fraction (HFpEF).
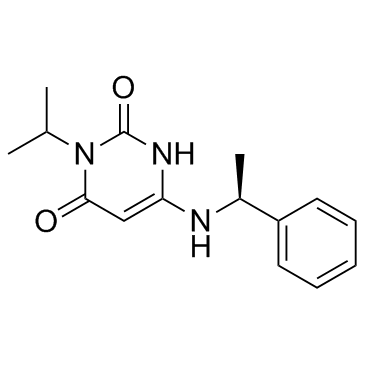
The chemical structure of mavacamten (picture source: chemsrc.com)
mavacamten (MYK-461) was developed by MyoKardia. On October 5, 2020, Bristol-Myers Squibb announced that it would acquire MyoKardia for USD 13.1 billion in cash and a 60% premium. On November 17, 2020, Bristol-Myers Squibb announced that the acquisition of MyoKardia has been successfully completed. This acquisition is Bristol-Myers Squibb’s second largest transaction after the US$74 billion acquisition of Celgene in 2019.
It is worth noting that on August 11, 2020, LianBio, which was incubated by the investment company Perceptive Advisors, was formally established. On the same day, it announced two important cooperations. One was to introduce BridgeBio Pharma’s product pipeline to China, and the other The project is to introduce Mavecamten from MyoKardia to China.
Bristol-Myers Squibb has high hopes for mavacamten. When it acquired MyoKardia, the company announced that mavacamten would become a pioneering drug for the treatment of HCM. The industry is also very optimistic about the business prospects of mavacamten. In December 2020, Evaluate Vantage, a pharmaceutical market research organization, released the “Top 10 New Drugs with Commercial Potential in 2021”. In this list, mavacamten ranked third. Evaluate Vantage predicts that mavacamten’s global sales in 2026 will be able to reach 2 billion US dollars.
(source:internet, reference only)
Disclaimer of medicaltrend.org



