Gastric cancer: Amgen’s FGFR2b targeted monoclonal antibody bemarituzumab
- A Single US$2.15-Million Injection to Block 90% of Cancer Cell Formation
- WIV: Prevention of New Disease X and Investigation of the Origin of COVID-19
- Why Botulinum Toxin Reigns as One of the Deadliest Poisons?
- FDA Approves Pfizer’s One-Time Gene Therapy for Hemophilia B: $3.5 Million per Dose
- Aspirin: Study Finds Greater Benefits for These Colorectal Cancer Patients
- Cancer Can Occur Without Genetic Mutations?
Gastric cancer: Amgen’s FGFR2b targeted monoclonal antibody bemarituzumab
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- What is the difference between Atorvastatin and Rosuvastatin?
- How long can the patient live after heart stent surgery?
Innovative therapy for gastric cancer: Amgen’s FGFR2b targeted monoclonal antibody bemarituzumab .
FDA granted Amgen’s FGFR2b targeted monoclonal antibody bemarituzumab breakthrough drug designation.
Amgen recently announced that the U.S. Food and Drug Administration (FDA) has granted breakthrough drug designation (BTD) for the first targeted therapy bemarituzumab, combined with an improved version of FOLFOX6 Chemotherapy (mFOLFOX6: fluoropyrimidine + leucovorin + oxaliplatin), first-line treatment based on FDA-approved companion diagnostic analysis showed that at least 10% of tumor cells overexpress fibroblast growth factor receptor 2b (FGFR2b), HER2 negative, and metastasis Patients with sexual or locally advanced gastric or gastroesophageal junction (GEJ) adenocarcinoma.
Data from the Phase 2 FIGH trial showed that in this patient population, bemarituzumab+mFOLFOX6 first-line treatment showed clinically significant and substantial benefits compared with placebo+mFOLFOX6, significantly prolonging progression-free survival (PFS) and overall Lifetime (OS).
bemarituzumab (anti-FGFR2b monoclonal antibody) is developed by Five Prime Therapeutics At the beginning of March this year, Amgen announced that it had reached a final agreement with Five Prime to acquire the latter’s shares in cash at a price of $38 per share, corresponding to a total equity of approximately $1.9 billion.
On April 16, Amgen announced that the acquisition has been successfully completed. In addition to bemarituzumab, Five Prime’s pipeline also complements Amgen’s tumor pipeline.
Stomach cancer
It is worth mentioning that bemarituzumab is the second oncology asset that Amgen has obtained BTD qualification in the past 6 months after sotorasib.
Globally, there are more than 1 million newly diagnosed gastric cancer cases each year, and the disease is particularly common in Asia. About 80%-85% of patients with advanced gastric cancer and GEJ cancer are HER2-negative, and about 30% of them show FGFR2b overexpression.
David M. Reese, MD, Executive Vice President of Amgen Research and Development, said: “The FIGHT trial is the first study to evaluate FGFR2b overexpression in targeted cancers.
As a first-line treatment, bemarituzumab has shown a key endpoint in patients with advanced gastric cancer or gastroesophageal cancer. Clinical significance.
Amgen looks forward to further research on the role of FGFR2b, and will continue to cooperate with regulatory agencies to take the next step to provide patients with this potential first-line treatment.”
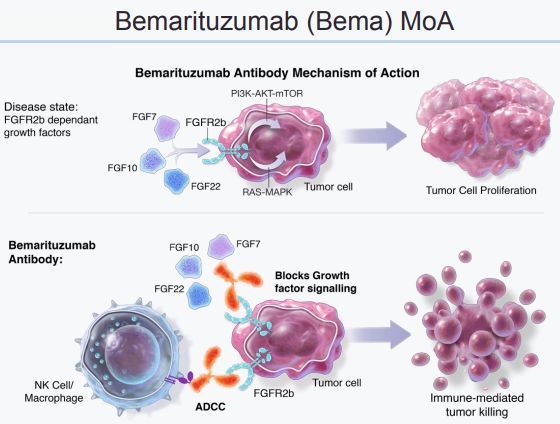
Mechanism of action of bemarituzumab (click on the picture to see a larger picture)
bemarituzumab is a first-in-class targeting antibody that prevents FGF from binding and activating FGFR2b, inhibits a variety of downstream pro-tumor signaling pathways, and may delay cancer progression.
Currently, bemarituzumab is being developed in gastric cancer and GEJ cancer as a targeted therapy for FGFR2b overexpressing tumors.
Five Prime is also evaluating the potential of bemarituzumab in other FGFR2b overexpressing tumors.
The FDA granted bemarituzumab BTD based on the results of this Phase 2 FIGH trial. This is a global, randomized, double-blind, placebo-controlled study in newly diagnosed fibroblast growth factor receptor 2b positive (FGFR2b+), non-HER2 positive, advanced gastric or gastroesophageal junction (GEJ) cancer patients To evaluate the efficacy and safety of bemarituzumab and placebo combined with chemotherapy (mFOLFOX6) for first-line treatment. The trial enrolled 155 patients in 15 countries in Asia, the European Union, and the United States, of which 77 were randomly assigned to the bemarituzumab treatment group (bemarituzumab+mFOLFOX6), and 78 patients were assigned to the placebo group (placebo+mFOLFOX6).
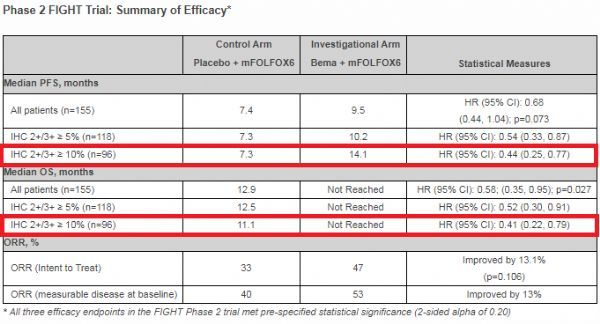
FIGHT clinical data (click on the picture to see a larger picture)
The results showed that in the patient population with at least 10% of tumor cells overexpressing FGFR2b, compared with the placebo group, patients in the bemarituzumab group showed progression-free survival (PFS) primary endpoints and overall survival (OS) secondary endpoints.
A clinically significant and substantial improvement. Additional analysis showed that treatment benefit was positively correlated with the percentage of FGFR2b+ tumor cells, confirming the importance of the FGFR2b target and the activity of bemarituzumab on this target.
The specific data are:
(1) In terms of the primary endpoint of PFS, the bemarituzumab group was significantly prolonged compared with placebo (median PFS: 14.1 months vs 7.3 months), and the risk of disease progression or death was reduced by 56% (HR=0.44; 95%) CI: 0.25-0.77).
(2) In terms of secondary endpoint OS, the bemarituzumab group was significantly prolonged compared with placebo (median OS: not reached [NR] vs 11.1 months), and the risk of death was reduced by 59% (HR=0.41; 95%CI: 0.22- 0.79).
(source:internet, reference only)
Disclaimer of medicaltrend.org



